Probenecidinhibitor of organic anion transport, MRP and pannexin-1 channel CAS# 57-66-9 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 57-66-9 | SDF | Download SDF |
| PubChem ID | 4911 | Appearance | Powder |
| Formula | C13H19NO4S | M.Wt | 285.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (350.43 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-(dipropylsulfamoyl)benzoic acid | ||
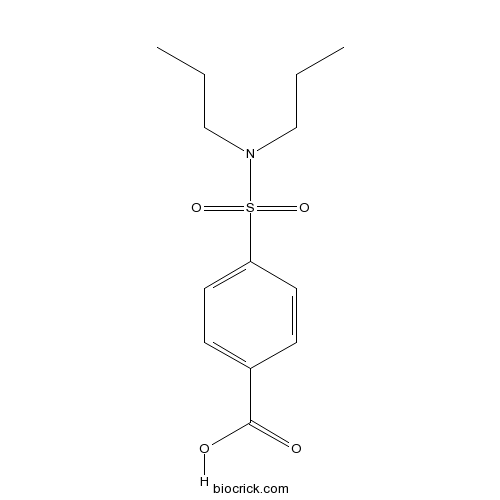
| SMILES | CCCN(CCC)S(=O)(=O)C1=CC=C(C=C1)C(=O)O | ||
| Standard InChIKey | DBABZHXKTCFAPX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H19NO4S/c1-3-9-14(10-4-2)19(17,18)12-7-5-11(6-8-12)13(15)16/h5-8H,3-4,9-10H2,1-2H3,(H,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of multidrug resistance-associated proteins (MRP). Inhibits organic acid transport in the kidney and other organs. Also exhibits inhibitory activity against pannexin 1 channels (IC50 ~ 150 μM). |

Probenecid Dilution Calculator

Probenecid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5043 mL | 17.5217 mL | 35.0435 mL | 70.0869 mL | 87.6086 mL |
| 5 mM | 0.7009 mL | 3.5043 mL | 7.0087 mL | 14.0174 mL | 17.5217 mL |
| 10 mM | 0.3504 mL | 1.7522 mL | 3.5043 mL | 7.0087 mL | 8.7609 mL |
| 50 mM | 0.0701 mL | 0.3504 mL | 0.7009 mL | 1.4017 mL | 1.7522 mL |
| 100 mM | 0.035 mL | 0.1752 mL | 0.3504 mL | 0.7009 mL | 0.8761 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Probenecid is an inhibitor of organic anion transport and MRP [1] [2]. Also, probenecid inhibited pannexin-1 channel with IC50 value of 150μM [3].
Multidrug resistance-associated proteins (MRPs) are ATP-binding cassette (ABC) transporters that transport various molecules across cellular membranes and are involved in multi-drug resistance.
Probenecid is an inhibitor of organic anion transport, MRP and pannexin-1 channel. In MRP-overexpressing HL60/AR and H69/AR tumor cell lines, probenecid reversed resistance to daunorubicin (DNR) and vincristine (VCR) in a concentration-dependent way [1]. In wild-type AML-2 cells, probenecid increased the MRP levels in a dose- and time-dependent way. In MRP-overexpressing AML cells, probenecid exhibited a significant chemosensitizing effect. These results suggested that probenecid functioned as an effective chemosensitizer of multidrug resistance (MDR) tumor cells but also an MRP activator [2].
In ischemia/reperfusion (I/R) injury rats, probenecid protected against CA1 neuronal death. Probenecid strengthened the upregulation of Hsp70 and inhibited the expression of calpain-1 and the released of cathepsin B. Also, probenecid inhibited the proliferation of astrocytes and microglia [4].
References:
[1]. Gollapudi S, Kim CH, Tran BN, et al. Probenecid reverses multidrug resistance in multidrug resistance-associated protein-overexpressing HL60/AR and H69/AR cells but not in P-glycoprotein-overexpressing HL60/Tax and P388/ADR cells. Cancer Chemother Pharmacol, 1997, 40(2): 150-158.
[2]. Kim HS, Min YD, Choi CH. Double-edged sword of chemosensitizer: increase of multidrug resistance protein (MRP) in leukemic cells by an MRP inhibitor probenecid. Biochem Biophys Res Commun, 2001, 283(1): 64-71.
[3]. Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol, 2008, 295(3): C761-767.
[4]. Wei R, Wang J, Xu Y, et al. Probenecid protects against cerebral ischemia/reperfusion injury by inhibiting lysosomal and inflammatory damage in rats. Neuroscience, 2015, 301: 168-177.
- Ethinyl Estradiol
Catalog No.:BCC3777
CAS No.:57-63-6
- Chlorotetracycline
Catalog No.:BCC8913
CAS No.:57-62-5
- Sucrose
Catalog No.:BCN5780
CAS No.:57-50-1
- Fructose
Catalog No.:BCN4969
CAS No.:57-48-7
- Esromiotin
Catalog No.:BCC8325
CAS No.:57-47-6
- Phenytoin
Catalog No.:BCC5070
CAS No.:57-41-0
- Benactyzine hydrochloride
Catalog No.:BCC8841
CAS No.:57-37-4
- Pentobarbital sodium salt
Catalog No.:BCC6231
CAS No.:57-33-0
- Phenobarbital sodium salt
Catalog No.:BCC6230
CAS No.:57-30-7
- Strychnine
Catalog No.:BCN4978
CAS No.:57-24-9
- Vincristine
Catalog No.:BCN5411
CAS No.:57-22-7
- Urea
Catalog No.:BCC8034
CAS No.:57-13-6
- Sulfaguanidine
Catalog No.:BCC4727
CAS No.:57-67-0
- Sulfamethazine
Catalog No.:BCC4942
CAS No.:57-68-1
- Progesterone
Catalog No.:BCN2198
CAS No.:57-83-0
- Testosterone propionate
Catalog No.:BCC9172
CAS No.:57-85-2
- Ergosterol
Catalog No.:BCN5787
CAS No.:57-87-4
- Cholesterol
Catalog No.:BCN2199
CAS No.:57-88-5
- α-Estradiol
Catalog No.:BCC7497
CAS No.:57-91-0
- (+)-Tubocurarine chloride
Catalog No.:BCC7496
CAS No.:57-94-3
- Europine
Catalog No.:BCN1976
CAS No.:570-19-4
- Stigmasta-5,8-dien-3-ol
Catalog No.:BCN5769
CAS No.:570-72-9
- Tricine
Catalog No.:BCN5337
CAS No.:5704-04-1
- Paprotrain
Catalog No.:BCC7978
CAS No.:57046-73-8
Symmetry assisted tuning of bending and brittle multi-component forms of probenecid.[Pubmed:28265636]
Chem Commun (Camb). 2017 Mar 16;53(23):3381-3384.
The mechanical flexibility known in the antihyperuricemia drug Probenecid has been extended into multi-component systems using co-formers with two donor or acceptor sites, in contrast to systems with a single H-bond acceptor that exhibit brittle behaviour. The piperazinium salt demonstrates that GRAS co-formers can be used to maintain mechanical flexibility with drug molecules.
Neurotrophic Effect of Asiatic acid, a Triterpene of Centella asiatica Against Chronic 1-Methyl 4-Phenyl 1, 2, 3, 6-Tetrahydropyridine Hydrochloride/Probenecid Mouse Model of Parkinson's disease: The Role of MAPK, PI3K-Akt-GSK3beta and mTOR Signalling Pathways.[Pubmed:28181071]
Neurochem Res. 2017 May;42(5):1354-1365.
Regulation of various signalling (Ras-MAPK, PI3K and AKT) pathways by augmented activity of neurotrophic factors (NTFs) could prevent or halt the progress of dopaminergic loss in Parkinson's disease (PD). Various in vitro and in vivo experimental studies indicated anti-parkinsonic potential of asiatic acid (AA), a pentacyclic triterpene obtained from Centella asiatica. So the present study is designed to determine the neurotrophic effect of AA against 1-methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine hydrochloride/Probenecid (MPTP/p) neurotoxicity in mice model of PD. AA treatment for 5 weeks significantly attenuated MPTP/p induced motor abnormalities, dopamine depletion and diminished expressions NTFs and tyrosine kinase receptors (TrKB). We further, revealed that AA treatment significantly inhibited the MPTP/p-induced phosphorylation of MAPK/P38 related proteins such as JNK and ERK. Moreover, AA treatment increased the phosphorylation of PI3K, Akt, GSK-3beta and mTOR, suggesting that AA activated PI3K/Akt/mTOR signalling pathway, which might be the cause of neuroprotection offered by AA. The present findings provided more elaborate in vivo evidences to support the neuroprotective effect of AA on dopaminergic neurons of chronic Parkinson's disease mouse model and the potential of AA to be developed as a possible new therapeutic target to treat PD.
Probenecid and N-Acetylcysteine Prevent Loss of Intracellular Glutathione and Inhibit Neuronal Death after Mechanical Stretch Injury In Vitro.[Pubmed:26830358]
J Neurotrauma. 2016 Oct 15;33(20):1913-1917.
Probenecid and N-acetylcysteine (NAC) can preserve intracellular levels of the vital antioxidant glutathione (GSH) via two distinct biochemical pathways. Probenecid inhibits transporter-mediated GSH efflux and NAC serves as a cysteine donor for GSH synthesis. We hypothesized that Probenecid and NAC alone would maintain intracellular GSH concentrations and inhibit neuronal death after traumatic stretch injury, and that the drugs in combination would produce additive effects. Sex-segregated rat primary cortical neurons were treated with Probenecid (100 muM) and NAC (50 muM), alone and in combination (Pro-NAC), then subjected to mechanical stretch (10s(-1) strain rate, 50% membrane deformation). At 24 h, both Probenecid and NAC inhibited trauma-induced intracellular GSH depletion, lactate dehydrogenase (LDH) release, and propidium iodide (PI) uptake in both XY- and XX-neurons. Combined Pro-NAC treatment was superior to Probenecid or NAC alone in maintenance of intracellular GSH and neuronal death assessed by PI uptake. Interestingly, caspase 3 activity 24 h after mechanical trauma was more prominent in XX-neurons, and treatment effects (Probenecid, NAC, and Pro-NAC) were observed in XX- but not XY-neurons; however, XY-neurons were ultimately more vulnerable to mechanical stretch-induced injury than their XX counterparts, as was evidenced by more neuronal death detected by LDH release and PI uptake. In addition, after stretch injury in HT22 hippocampal cells, both NAC and Probenecid were highly effective at reducing oxidative stress detected by dichlorofluorescein fluorescence. These in vitro data support further testing of this drug combination in models of traumatic neuronal injury in vivo.
Mulberry fruit ameliorates Parkinson's-disease-related pathology by reducing alpha-synuclein and ubiquitin levels in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid model.[Pubmed:27741433]
J Nutr Biochem. 2017 Jan;39:15-21.
Mulberry fruit, which has been long used in traditional oriental medicine, was reported to ameliorate motor dysfunction and dopaminergic neuronal degeneration via antioxidant and antiapoptotic effects in an animal model of Parkinson's disease (PD). More than 95% of PD patients exhibit nonmotor problems such as olfactory dysfunction and gastrointestinal constipation, which are generally considered to be early symptoms of PD. However, few studies have actually examined potential drugs to treat early PD symptoms. The present study examined the protective effects of mulberry fruit extract (ME) against neurotoxicity in a 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine/Probenecid (MPTP/p) model of early PD. MPTP/p model was developed by systemic administration with MPTP (25 mg/kg) and Probenecid (250 mg/kg) over 5 weeks. The behavioral studies showed that treatment of mice with ME significantly improved PD-related nonmotor symptoms as well as motor impairment, demonstrated by utilizing the olfactory, pole, rotarod and open field tests. In addition, immunohistochemical analysis indicated that ME exhibits the protective effects against dopaminergic neuronal damage induced by MPTP/p in the substantia nigra and striatum. Moreover, by using Western blot analysis, we found that treatment with ME inhibited the up-regulation of alpha-synuclein and ubiquitin, well known as composition of Lewy bodies in the substantia nigra and striatum of the MPTP/p mice. Taken together, these data suggest that ME may have therapeutic potential for preventing PD.
Function and expression of ATP-binding cassette transporters in cultured human Y79 retinoblastoma cells.[Pubmed:20190417]
Biol Pharm Bull. 2010;33(3):504-11.
The aim of this study was to reveal the expression and function of P-glycoprotein and multidrug resistance-associated proteins (MRP), members of the ATP-binding cassette (ABC) superfamily of drug transporters, in cultured human Y79 retinoblastoma cells. ABC transporter mRNA expression was evaluated by conventional reverse transcription-polymerase chain reaction (RT-PCR) and real-time PCR analyses. Cellular accumulation of rhodamine 123 (P-glycoprotein substrate), calcein (MRP substrate), and doxorubicin (P-glycoprotein/MRP substrate) was analyzed by fluorometry. Conventional RT-PCR analysis showed the expression of multidrug resistance 1 (MDR1), MRP1, MRP2 and lung resistance-related protein (LRP) mRNAs. Real-time RT-PCR analysis revealed that the expression levels of the MDR1 and MRP2 genes in Y79 cells were much lower than those in human intestinal cell line Caco-2, while the expression level of MRP1 was higher than that in Caco-2 cells. The accumulation of rhodamine 123 was not enhanced by verapamil or reversin 205, inhibitors of P-glycoprotein, indicating no function of P-glycoprotein in Y79 cells. The accumulation of calcein was significantly increased by various MRP inhibitors including Probenecid, indicating that MRP functions in Y79 cells. The accumulation of doxorubicin was increased in the presence of metabolic inhibitors (10 mM 2-deoxyglucose and 5 mM sodium azide). However, most MRP inhibitors such as Probenecid and indomethacin did not affect doxorubicin accumulation, while cyclosporin A and taclorimus significantly increased doxorubicin accumulation. These results suggest that MRP, but not P-glycoprotein, functions in Y79 cells, and that the efflux of doxorubicin from Y79 cells may be due to an ATP-dependent transporter, which has not been identified yet.
Probenecid, a gout remedy, inhibits pannexin 1 channels.[Pubmed:18596212]
Am J Physiol Cell Physiol. 2008 Sep;295(3):C761-7.
Probenecid is a well-established drug for the treatment of gout and is thought to act on an organic anion transporter, thereby affecting uric acid excretion in the kidney by blocking urate reuptake. Probenecid also has been shown to affect ATP release, leading to the suggestion that ATP release involves an organic anion transporter. Other pharmacological evidence and the observation of dye uptake, however, suggest that the nonvesicular release of ATP is mediated by large membrane channels, with pannexin 1 being a prominent candidate. In the present study we show that Probenecid inhibited currents mediated by pannexin 1 channels in the same concentration range as observed for inhibition of transport processes. Probenecid did not affect channels formed by connexins. Thus Probenecid allows for discrimination between channels formed by connexins and pannexins.
Glutathione export during apoptosis requires functional multidrug resistance-associated proteins.[Pubmed:17374608]
J Biol Chem. 2007 May 11;282(19):14337-47.
GSH is released in cells undergoing apoptosis, and the present study indicates that the multidrug resistance-associated proteins (MRPs/ABCC) are responsible for this GSH release. Jurkat cells released approximately 75-80% of their total intracellular GSH during both Fas antibody- and staurosporine-induced apoptosis. In contrast, Raji cells, a lymphocyte cell line that is deficient in phosphatidylserine externalization, did not release GSH during apoptosis, and other apoptotic features appeared more slowly in these cells. Jurkat and Raji cell lines expressed comparable MRP and OATP/SLCO (organic anion-transporting polypeptide) mRNA levels, and MRP1 protein levels; however, differences existed in MRP1 localization and function. In Jurkat cells, MRP1 was largely localized to the plasma membrane, and these cells exported the MRP substrate calcein. Calcein release was enhanced during apoptosis. In contrast, Raji cells had little MRP1 at the plasma membrane and did not export calcein under basal or apoptotic conditions, indicating that these cells lack functional MRPs at the plasma membrane. GSH release in Jurkat cells undergoing apoptosis was inhibited by the organic anion transport inhibitors MK571, sulfinpyrazone, and Probenecid, supporting a role for the MRP transporters in this process. Furthermore, when MRP1 expression was decreased with RNA interference, GSH release was lower under both basal and apoptotic conditions, providing direct evidence that MRP1 is involved in GSH export.
Clinical pharmacokinetics of probenecid.[Pubmed:7011657]
Clin Pharmacokinet. 1981 Mar-Apr;6(2):135-51.
A review of the clinical applications and of the disposition of Probenecid in man, including drug interactions, is presented. Probenecid is the classical competitive inhibitor of organic acid transport in the kidney and other organs. There are 2 primary clinical uses for Probenecid: as a uricosuric agent in the treatment of chronic gout and as an adjunct to enhance blood levels of antibiotics (such as penicillins and cephalosporins). Adsorption of Probenecid is essentially complete following oral administration. The drug is extensively metabolised by glucuronide conjugation and by oxidation of the alkyl side chains; oxidation of the aromatic ring does not occur. The half-life of Probenecid in plasma (4 to 12 hours) is dose-dependent. Renal excretion is the major route of elimination of the metabolites; excretion of the parent drug is minimal and is dependent on urinary pH. Probenecid and its oxidised metabolites are extensively bound to plasma proteins, mainly to albumin. Tissue concentrations (based on animal studies) are generally lower than plasma concentrations. Most of the drug-drug interactions involving Probenecid are due to an effect on the kidney-block of transport of acidic drugs. Similarly Probenecid affects the tubular secretion of a number of acidic endogenous substances by the kidney. Probenecid is also involved in the block of transport of acidic metabolites of catecholamines, for example homovanillic and hydroxyindoleacetic acids, in the brain. There are a number of analytical procedures for the assay of Probenecid. These are based on spectrophotometry, spectrofluorometry, gas and liquid chromatography and radioimmunoassay.


