RegorafenibInhibitor of VEGFR/PDGFR/FGFR/mutant kit/RET/Raf-1 CAS# 755037-03-7 |
- TAK-593
Catalog No.:BCC5142
CAS No.:1005780-62-0
- ZM323881
Catalog No.:BCC2073
CAS No.:193001-14-8
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- Motesanib
Catalog No.:BCC1776
CAS No.:453562-69-1
- Pazopanib Hydrochloride
Catalog No.:BCC3708
CAS No.:635702-64-6
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
Quality Control & MSDS
Number of papers citing our products

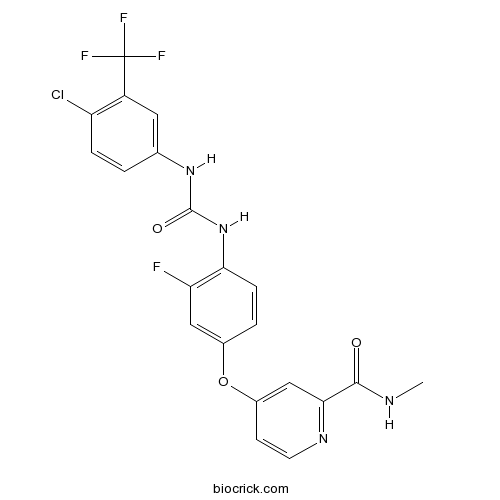
Chemical structure
3D structure
| Cas No. | 755037-03-7 | SDF | Download SDF |
| PubChem ID | 11167602 | Appearance | Powder |
| Formula | C21H15ClF4N4O3 | M.Wt | 482.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BAY 73-4506 | ||
| Solubility | DMSO : ≥ 260 mg/mL (538.50 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide | ||
| SMILES | CNC(=O)C1=NC=CC(=C1)OC2=CC(=C(C=C2)NC(=O)NC3=CC(=C(C=C3)Cl)C(F)(F)F)F | ||
| Standard InChIKey | FNHKPVJBJVTLMP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Regorafenib (BAY 73-4506) is a multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFRβ, Kit, RET and Raf-1 with IC50 of 13 nM/4.2 nM/46 nM, 22 nM, 7 nM, 1.5 nM and 2.5 nM, respectively. | ||||||
| Targets | VEGFR1/2/3 | PDGFRβ | Kit | RET | Raf-1 | ||
| IC50 | 13 nM/4.2 nM/46 nM | 22 nM | 7 nM | 1.5 nM | 2.5 nM | ||
| Cell experiment: [1] | |
| Cell lines | PLC/PRF/5 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 1 μM, 72 hours for migration assay 5 μM, 24 hours for invasion assay |
| Applications | For the migration assay, PLC/PRF/5 cells were treated with the drugs and microscopically analyzed at the time of the scratch (T0) and after 48 and 72 hours. For the invasion assay, invading PLC/PRF/5 cells were treated with different drug concentrations (0.5, 1, 2.5 and 5 μM). Invasion was calculated as a percentage of the invading drug-treated cells compared to drug-untreated control cells. Regorafenib inhibited HCC cell migration in both AFP-positive and AFP-negative cells at the same low concentration range as inhibited AFP levels. Similar results were found in a cell invasion assay, at almost identical drug concentrations. |
| Animal experiment: [2] | |
| Animal models | Female athymic NCr nu/nu mice injected with Colo-205, MDA-MB-231 or 786-O xenografts |
| Dosage form | Oral administration; 100, 30, 10, and 3 mg/kg |
| Application | Regorafenib dosed qd orally inhibited tumor growth in a dose-dependent manner in multiple xenograft models, including models derived from CRC (Colo-205), BC (MDA-MB-231) and RCC (786-O) tumors. Regorafenib effectively inhibited growth of the Colo-205 xenografts in the dose range of 10-100 mg/kg, reaching a TGI of about 75% at day 14 at the 10 mg/kg dose. A slow regrowth was observed at all doses when treatment was terminated after 9 days. In the MDA-MB-231 model, regorafenib was highly efficacious at a dose as low as 3 mg/kg, resulting in a significant TGI of 81%, which increased to ~ 93% at doses of 10 and 30 mg/kg, where tumor stasis was reached. Regorafenib also very efficiently inhibited the growth of the 786-O RCC model. TGI >90% was observed at the end of a 21-day dosing period with regorafenib 10 and 30 mg/kg. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Carr B I, D'Alessandro R, Refolo M G, et al. Effects of low concentrations of regorafenib and sorafenib on human HCC cell AFP, migration, invasion, and growth in vitro. Journal of cellular physiology, 2013, 228(6): 1344-1350. [2] Wilhelm S M, Dumas J, Adnane L, et al. Regorafenib (BAY 73‐4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. International Journal of Cancer, 2011, 129(1): 245-255. | |

Regorafenib Dilution Calculator

Regorafenib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0713 mL | 10.3563 mL | 20.7125 mL | 41.425 mL | 51.7813 mL |
| 5 mM | 0.4143 mL | 2.0713 mL | 4.1425 mL | 8.285 mL | 10.3563 mL |
| 10 mM | 0.2071 mL | 1.0356 mL | 2.0713 mL | 4.1425 mL | 5.1781 mL |
| 50 mM | 0.0414 mL | 0.2071 mL | 0.4143 mL | 0.8285 mL | 1.0356 mL |
| 100 mM | 0.0207 mL | 0.1036 mL | 0.2071 mL | 0.4143 mL | 0.5178 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Regorafenib (BAY 73-4506) is a novel and orally active multikinase inhibitor of receptor tyrosine kinases VEGFR1, VEGFR2, VEGFR3, PDGFRβ, Kit, RET, Raf-1, B-RAF and B-RAFV600E with IC50 values of 13 nM/4.2 nM/46 nM, 22 nM, 7 nM, 1.5 nM, 2.5 nM, 28 nM and 19 nM [1].
VEGFR1/2/3 are vascular endothelial growth factor receptor plays an important role in the formation of normal and tumor vasculature. platelet-derived growth factor receptor-β (PDGFRβ) is a receptor for the platelet-derived growth factor family. Kit, RET and B-RAF are both receptor tyrosine kinases that encoded by proto-oncogenes [1].
Regorafenib (BAY 73-4506) is a novel and orally active receptor tyrosine kinases inhibitor. In NIH-3T3/VEGFR2 cells, regorafenib potently inhibited VEGFR2 autophosphorylation with IC50 value of 3 nM. Regorafenib also inhibited TIE2 and PDGFR-β autophosphorylation with IC50 values of 31 and 90 nM. Regorafenib potently inhibited KITK642E and RETC634W with IC50 values of ~20 and ~10 nM, respectively. In addition, regorafenib inhibited the proliferation of VEGF165-stimulated HUVECs with IC50 value of ~3 nM [1].
In GS9L glioblastoma xenografted rat model, regorafenib administered orally at 10 mg/kg significantly reduced the extravasation of Gadomer in the vasculature. In various preclinical human xenograft mice models, regorafenib exhibited potent dose-dependent tumor growth inhibition (TGI) [1]. In murine metastatic colorectal cancer (CRC) liver metastasis model, regorafenib significantly delayed disease progression by inhibiting the growth of liver metastases and preventing the formation of new metastases in other organs [2].
References:
[1]. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer, 2011, 129(1): 245-255.
[2]. Schmieder R, Hoffmann J, Becker M, et al. Regorafenib (BAY 73-4506): antitumor and antimetastatic activities in preclinical models of colorectal cancer. Int J Cancer, 2014, 135(6): 1487-1496.
- N-Methylnuciferine
Catalog No.:BCN3971
CAS No.:754919-24-9
- CGP 37157
Catalog No.:BCC6943
CAS No.:75450-34-9
- Moxonidine
Catalog No.:BCC2142
CAS No.:75438-57-2
- EHT 1864
Catalog No.:BCC6075
CAS No.:754240-09-0
- Boc-Asp(OBzl)-OH
Catalog No.:BCC2608
CAS No.:7536-58-5
- Boc-Asn-OH
Catalog No.:BCC3071
CAS No.:7536-55-2
- Indacaterol Maleate
Catalog No.:BCC4358
CAS No.:753498-25-8
- Lovastatin
Catalog No.:BCN1060
CAS No.:75330-75-5
- H-Leucinol
Catalog No.:BCC2725
CAS No.:7533-40-6
- H-Pro-NH2
Catalog No.:BCC3018
CAS No.:7531-52-4
- Kukoamine A
Catalog No.:BCN3835
CAS No.:75288-96-9
- HEPES Sodium salt
Catalog No.:BCC7591
CAS No.:75277-39-3
- BI 2536
Catalog No.:BCC2081
CAS No.:755038-02-9
- BI6727 (Volasertib)
Catalog No.:BCC3886
CAS No.:755038-65-4
- Flupirtine maleate
Catalog No.:BCC4456
CAS No.:75507-68-5
- Cedrin
Catalog No.:BCN4748
CAS No.:75513-81-4
- Nilvadipine
Catalog No.:BCC3799
CAS No.:75530-68-6
- Moxonidine hydrochloride
Catalog No.:BCC5163
CAS No.:75536-04-8
- Dencichin
Catalog No.:BCN2555
CAS No.:7554-90-7
- Ingenol 3-Angelate
Catalog No.:BCN2961
CAS No.:75567-37-2
- 20-Deoxyingenol 3-angelate
Catalog No.:BCN6642
CAS No.:75567-38-3
- Sodium phosphate dibasic
Catalog No.:BCC7585
CAS No.:7558-79-4
- Sodium phosphate monobasic
Catalog No.:BCC8033
CAS No.:7558-80-7
- alpha-Tocopherolquinone
Catalog No.:BCN4305
CAS No.:7559-04-8
REGOSARC: Regorafenib versus placebo in doxorubicin-refractory soft-tissue sarcoma-A quality-adjusted time without symptoms of progression or toxicity analysis.[Pubmed:28295221]
Cancer. 2017 Jun 15;123(12):2294-2302.
BACKGROUND: In a placebo-controlled, randomized phase 2 trial (ClinicalTrials.gov identifier NCT01900743), Regorafenib improved progression-free survival (PFS) for patients with doxorubicin-pretreated advanced nonadipocytic sarcoma. A quality-adjusted time without symptoms of progression or toxicity (Q-TWiST) post hoc exploratory analysis was applied to provide an integrated measure of its clinical benefit. METHODS: In the base-case analysis, each patient's overall survival (OS) was partitioned into 3 mutually exclusive health states: the time with a grade 3 or 4 adverse event (TOX), the time without symptoms of disease or grade 3 or 4 toxicity from treatment, and the time after tumor progression or relapse. The time spent in each state was weighted with a health-state utility associated with that state and was summed to calculate the Q-TWiST. The stability of the base-case analysis was explored with several sensitivity analyses. RESULTS: In nonadipocytic sarcoma, the PFS was (4.0 months [2.6-5.5 months] with Regorafenib vs 1.0 month [0.9-1.8 months] with a placebo; hazard ratio, 0.36 [0.25-0.53]; P < .0001); the OS was 13.4 months (8.6-17.3 months) with Regorafenib and 9.0 months (6.8-12.5 months) with a placebo (hazard ratio, 0.67 [0.44-1.02]). With the classic definition of TOX (including all grade 3 and 4 clinical adverse events), the Q-TWiSTs were 8.0 months (7.0-9.0 months) with Regorafenib and 5.7 months (4.9-6.4 months) with a placebo (P < .001). CONCLUSIONS: For patients with doxorubicin-pretreated soft-tissue sarcoma, Regorafenib significantly improved quality-adjusted survival in comparison with a placebo. Cancer 2017;123:2294-2302. (c) 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Regorafenib overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter in colorectal cancer: In vitro and in vivo study.[Pubmed:28302530]
Cancer Lett. 2017 Jun 28;396:145-154.
Chemotherapeutic multidrug resistance (MDR) is a significant challenge to overcome in clinic practice. Several mechanisms contribute to MDR, one of which is the augmented drug efflux induced by the upregulation of ABCB1 in cancer cells. Regorafenib, a multikinase inhibitor targeting the RAS/RAF/MEK/ERK pathway, was approved by the FDA to treat metastatic colorectal cancer and gastrointestinal stromal tumors. We investigated whether and how Regorafenib overcame MDR mediated by ABCB1. The results showed that Regorafenib reversed the ABCB1-mediated MDR and increased the accumulation of [(3)H]-paclitaxel in ABCB1-overexpressing cells by suppressing efflux activity of ABCB1, but not altering expression level and localization of ABCB1. Regorafenib inhibited ATPase activity of ABCB1. In mice bearing resistant colorectal tumors, Regorafenib raised the intratumoral concentration of paclitaxel and suppressed the growth of resistant colorectal tumors. But Regorafenib did not induce cardiotoxicity/myelosuppression of paclitaxel in mice. Strategy to reposition one FDA-approved anticancer drug Regorafenib to overcome the resistance of another FDA-approved, widely used chemotherapeutic paclitaxel, may be a promising direction for the field of adjuvant chemotherapy. This study provides clinical rationale for combination of conventional chemotherapy and targeted anticancer agents.
Cost-Effectiveness Analysis of Regorafenib for Gastrointestinal Stromal Tumour (GIST) in Germany.[Pubmed:28361439]
Clin Drug Investig. 2017 Jun;37(6):525-533.
BACKGROUND: No study has compared the cost-effectiveness of active treatment options for unresectable or metastatic gastrointestinal stromal tumours in patients who progressed on or are intolerant to prior treatment with imatinib and sunitinib. The aim of this study was to estimate the cost-effectiveness of Regorafenib compared to imatinib rechallenge in this setting in Germany. METHODS: Hazard ratios for progression-free (PFS) and overall survival (OS) with Regorafenib versus imatinib rechallenge were estimated by indirect comparison. A state distribution model was used to simulate progression, mortality and treatment costs over a lifetime horizon. Drug acquisition costs and utilities were derived from clinical trial data and published literature; non-drug costs were not included. The outcomes measured were treatment costs, life-years (LYs) and quality-adjusted life-years (QALYs). RESULTS: The indirect comparison suggested that median PFS and OS were longer with Regorafenib compared to imatinib but results were not statistically significant. Regorafenib versus imatinib rechallenge was estimated to have hazard ratios of 0.58 (95% CI 0.31-1.11) for PFS and 0.77 (95% CI 0.34-1.77) for OS, with substantial uncertainty due to the rarity of the disease and small number of patients within the trials. Regorafenib treatment per patient over a lifetime horizon provided an additional 0.61 LYs and 0.42 QALYs over imatinib rechallenge, with additional direct drug costs of euro8,773. The incremental cost-effectiveness ratio was euro21,127 per QALY gained. At a cost-effectiveness threshold of euro50,000 per QALY, Regorafenib had a 67% probability of being cost-effective. CONCLUSION: Based on the currently available clinical data, this analysis suggests that Regorafenib is cost-effective compared with imatinib rechallenge in Germany.


