TCB-2Potent, high affinity 5-HT2A agonist CAS# 912342-28-0 |
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 912342-28-0 | SDF | Download SDF |
| PubChem ID | 16086372 | Appearance | Powder |
| Formula | C11H15Br2NO2 | M.Wt | 353.05 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water and to 100 mM in DMSO | ||
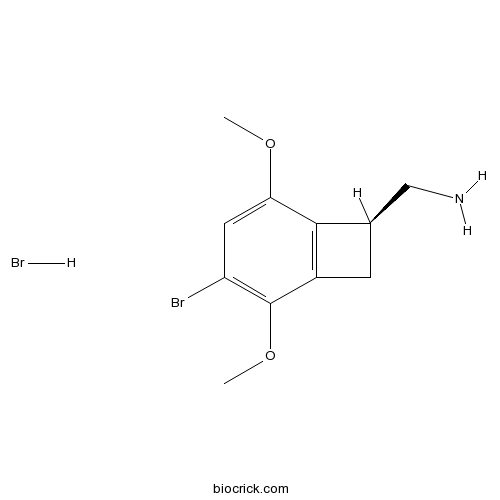
| Chemical Name | [(7S)-3-bromo-2,5-dimethoxy-7-bicyclo[4.2.0]octa-1(6),2,4-trienyl]methanamine;hydrobromide | ||
| SMILES | COC1=CC(=C(C2=C1C(C2)CN)OC)Br.Br | ||
| Standard InChIKey | TYMMXVZAUGQKRF-FYZOBXCZSA-N | ||
| Standard InChI | InChI=1S/C11H14BrNO2.BrH/c1-14-9-4-8(12)11(15-2)7-3-6(5-13)10(7)9;/h4,6H,3,5,13H2,1-2H3;1H/t6-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity 5-HT2A receptor agonist (Ki values are 0.73 and 0.75 nM for rat and human receptors respectively). Potently stimulates IP3 accumulation in NIH3T3 cells stably expressing rat 5-HT2A receptors (EC50 = 36 nM). Induces head twitches and hypothermia in mice following i.p. administration. |

TCB-2 Dilution Calculator

TCB-2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8325 mL | 14.1623 mL | 28.3246 mL | 56.6492 mL | 70.8115 mL |
| 5 mM | 0.5665 mL | 2.8325 mL | 5.6649 mL | 11.3298 mL | 14.1623 mL |
| 10 mM | 0.2832 mL | 1.4162 mL | 2.8325 mL | 5.6649 mL | 7.0811 mL |
| 50 mM | 0.0566 mL | 0.2832 mL | 0.5665 mL | 1.133 mL | 1.4162 mL |
| 100 mM | 0.0283 mL | 0.1416 mL | 0.2832 mL | 0.5665 mL | 0.7081 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Karavilagenin A
Catalog No.:BCN4455
CAS No.:912329-03-4
- Spantide I
Catalog No.:BCC5808
CAS No.:91224-37-2
- Noscapine HCl
Catalog No.:BCC3819
CAS No.:912-60-7
- H-Met-OtBu.HCl
Catalog No.:BCC2996
CAS No.:91183-71-0
- Ophiopogonin C
Catalog No.:BCN5379
CAS No.:911819-08-4
- Lucyoside B
Catalog No.:BCN7811
CAS No.:91174-19-5
- Chrysothol
Catalog No.:BCN4454
CAS No.:911714-91-5
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
- Terbinafine
Catalog No.:BCC3865
CAS No.:91161-71-6
- Infractin
Catalog No.:BCN3652
CAS No.:91147-07-8
- SLx-2119
Catalog No.:BCC1954
CAS No.:911417-87-3
- EC 144
Catalog No.:BCC5600
CAS No.:911397-80-3
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
- Veliparib dihydrochloride
Catalog No.:BCC2076
CAS No.:912445-05-7
- SAG
Catalog No.:BCC6390
CAS No.:912545-86-9
- Melilotigenin B
Catalog No.:BCN4456
CAS No.:91269-84-0
- Sarafloxacin HCl
Catalog No.:BCC4713
CAS No.:91296-87-6
- AT13387
Catalog No.:BCC2122
CAS No.:912999-49-6
- ELN441958
Catalog No.:BCC6452
CAS No.:913064-47-8
- LY 2087101
Catalog No.:BCC7869
CAS No.:913186-74-0
- Almorexant hydrochloride
Catalog No.:BCC5123
CAS No.:913358-93-7
- AMG-458
Catalog No.:BCC3721
CAS No.:913376-83-7
- Brexpiprazole
Catalog No.:BCC4118
CAS No.:913611-97-9
- 1''-Hydroxyerythrinin C
Catalog No.:BCN4066
CAS No.:913690-46-7
Activation of 5-HT2A receptors by TCB-2 induces recurrent oscillatory burst discharge in layer 5 pyramidal neurons of the mPFC in vitro.[Pubmed:24844635]
Physiol Rep. 2014 May 20;2(5). pii: 2/5/e12003.
The medial prefrontal cortex (mPFC) is a region of neocortex that plays an integral role in several cognitive processes which are abnormal in schizophrenic patients. As with other cortical regions, large-bodied layer 5 pyramidal neurons serve as the principle subcortical output of microcircuits of the mPFC. The coexpression of both inhibitory serotonin 5-HT1A receptors on the axon initial segments, and excitatory 5-HT2A receptors throughout the somatodendritic compartments, by layer 5 pyramidal neurons allows serotonin to provide potent top-down regulation of input-output relationships within cortical microcircuits. Application of 5-HT2A agonists has previously been shown to enhance synaptic input to layer 5 pyramidal neurons, as well as increase the gain in neuronal firing rate in response to increasing depolarizing current steps. Using whole-cell patch-clamp recordings obtained from layer 5 pyramidal neurons of the mPFC of C57/bl6 mice, the aim of our present study was to investigate the modulation of long-term spike trains by the selective 5-HT2A agonist TCB-2. We found that in the presence of synaptic blockers, TCB-2 induced recurrent oscillatory bursting (ROB) after 15-20 sec of tonic spiking in 7 of the 14 cells. In those seven cells, ROB discharge was accurately predicted by the presence of a voltage sag in response to a hyperpolarizing current injection. This effect was reversed by 5-10 min of drug washout and ROB discharge was inhibited by both synaptic activity and coapplication of the 5-HT2A/2C antagonist ketanserin. While the full implications of this work are not yet understood, it may provide important insight into serotonergic modulation of cortical networks.
The serotonin 5-HT(2A) receptor agonist TCB-2: a behavioral and neurophysiological analysis.[Pubmed:19823806]
Psychopharmacology (Berl). 2010 Sep;212(1):13-23.
RATIONALE: There are few reports on the high-affinity 5-HT(2A) agonist (4-Bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide (TCB-2). OBJECTIVES: Here we provide the first behavioral and neurophysiological profile of TCB-2 in C57BL/6J mice, with direct comparisons to the 5-HT(2A/2C) agonist (+/-)-2,5-dimethoxy-4-iodophenyl-2-aminopropane (DOI), in addition to determinations of 5-HT(2A) mediation via pretreatment with the selective 5-HT(2A) antagonist MDL 11,939. RESULTS: In a dose-dependent manner, TCB-2 induced head twitches, decreased food consumption in food-deprived mice, induced hypothermia, and increased corticosterone levels, with no effects on locomotor activity or anxiety-like behaviors in the open field. Similar effects were observed in side-by-side dose-response comparisons with DOI; although at the highest dose tested (5.0 mg/kg), TCB-2 induced significantly fewer head twitches, and a significantly enhanced hypothermic response, versus DOI. Pretreatment with MDL 11,939 blocked head twitches and temperature change following TCB-2 and DOI, confirming 5-HT(2A) mediation of these responses. Although MDL 11,939 pretreatment blocked DOI-induced suppression of feeding, MDL 11,939 had no effect on TCB-2-induced suppression of feeding. Previous studies show that 5-HT(2A) function is altered by changes in serotonin transporter (SERT) expression and function. In SERT knockout (-/-) mice, TCB-2-induced head twitches and hypothermia were greatly diminished compared to SERT wild-type (+/+) mice. CONCLUSIONS: The current studies are important, as they are the first to assess the effects of TCB-2 in mice, and are among the first to report the behavioral and neurophysiological effects of this conformationally restricted phenethylamine analog compound, which has 65-fold greater effects on signaling via the phosphoinositide versus arachidonic acid pathways.
Expression and function of serotonin 2A and 2B receptors in the mammalian respiratory network.[Pubmed:21789169]
PLoS One. 2011;6(7):e21395.
Neurons of the respiratory network in the lower brainstem express a variety of serotonin receptors (5-HTRs) that act primarily through adenylyl cyclase. However, there is one receptor family including 5-HT(2A), 5-HT(2B), and 5-HT(2C) receptors that are directed towards protein kinase C (PKC). In contrast to 5-HT(2A)Rs, expression and function of 5-HT(2B)Rs within the respiratory network are still unclear. 5-HT(2B)R utilizes a Gq-mediated signaling cascade involving calcium and leading to activation of phospholipase C and IP3/DAG pathways. Based on previous studies, this signal pathway appears to mediate excitatory actions on respiration. In the present study, we analyzed receptor expression in pontine and medullary regions of the respiratory network both at the transcriptional and translational level using quantitative RT-PCR and self-made as well as commercially available antibodies, respectively. In addition we measured effects of selective agonists and antagonists for 5-HT(2A)Rs and 5-HT(2B)Rs given intra-arterially on phrenic nerve discharges in juvenile rats using the perfused brainstem preparation. The drugs caused significant changes in discharge activity. Co-administration of both agonists revealed a dominance of the 5-HT(2B)R. Given the nature of the signaling pathways, we investigated whether intracellular calcium may explain effects observed in the respiratory network. Taken together, the results of this study suggest a significant role of both receptors in respiratory network modulation.
1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists.[Pubmed:16970404]
J Med Chem. 2006 Sep 21;49(19):5794-803.
A series of conformationally restricted analogues of the hallucinogenic phenethylamine 1 (2,5-dimethoxy-4-bromophenethylamine, 2C-B) was synthesized to test several hypotheses concerning the bioactive conformation of phenethylamine ligands upon binding to the 5-HT(2A) receptor. These benzocycloalkane analogues were assayed for their receptor binding affinity and ability to activate downstream signaling pathways, and one exceptional compound was selected for testing in an in vivo drug discrimination model of hallucinogenesis. All compounds were examined in silico by virtual docking into a homology model of the 5-HT(2A) receptor. On the basis of these docking experiments, it was predicted that the R enantiomer of benzocyclobutene analogue 2 would be the most potent. Subsequent chemical resolution and X-ray crystallography confirmed this prediction, as (R)-2 proved to be equipotent to LSD in rats trained to discriminate LSD from saline. Thus, we propose that the conformation of 2 mimics the active binding conformation of the more flexible phenethylamine type hallucinogens. In addition, (R)-2 is one of the most potent and selective compounds yet discovered in the in vivo drug discrimination assay. Further, 2 was found to be a functionally selective agonist at the 5-HT(2A) receptor, having 65-fold greater potency in stimulating phosphoinositide turnover than in producing arachidonic acid release. If hallucinogenic effects are correlated with arachidonic acid production, such functionally selective 5-HT(2A) receptor agonists may lack the intoxicating properties of hallucinogens such as LSD.


