LY 2087101Potentiator of nAChRs CAS# 913186-74-0 |
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- GW3965
Catalog No.:BCC1612
CAS No.:405911-09-3
- GW3965 HCl
Catalog No.:BCC3790
CAS No.:405911-17-3
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 913186-74-0 | SDF | Download SDF |
| PubChem ID | 11964458 | Appearance | Powder |
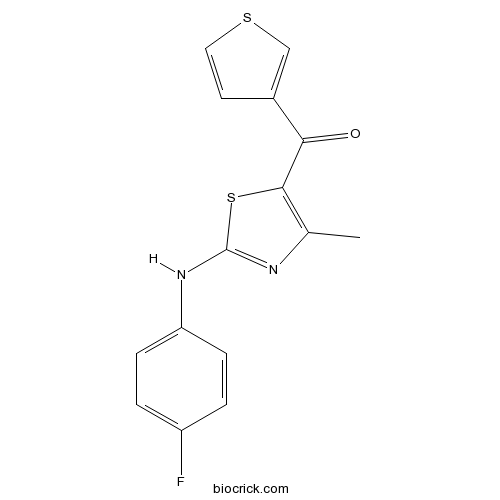
| Formula | C15H11FN2OS2 | M.Wt | 318.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in ethanol | ||
| Chemical Name | [2-(4-fluoroanilino)-4-methyl-1,3-thiazol-5-yl]-thiophen-3-ylmethanone | ||
| SMILES | CC1=C(SC(=N1)NC2=CC=C(C=C2)F)C(=O)C3=CSC=C3 | ||
| Standard InChIKey | PEAMDZVDNYENPN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H11FN2OS2/c1-9-14(13(19)10-6-7-20-8-10)21-15(17-9)18-12-4-2-11(16)3-5-12/h2-8H,1H3,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Allosteric potentiator of α7, α4β2 and α4β4 nAChRs; displays selectivity against α3β4 nAChRs. Thought to potentiate agonist-evoked α7 responses by binding within the nAChR transmembrane region. |

LY 2087101 Dilution Calculator

LY 2087101 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1408 mL | 15.704 mL | 31.408 mL | 62.816 mL | 78.5201 mL |
| 5 mM | 0.6282 mL | 3.1408 mL | 6.2816 mL | 12.5632 mL | 15.704 mL |
| 10 mM | 0.3141 mL | 1.5704 mL | 3.1408 mL | 6.2816 mL | 7.852 mL |
| 50 mM | 0.0628 mL | 0.3141 mL | 0.6282 mL | 1.2563 mL | 1.5704 mL |
| 100 mM | 0.0314 mL | 0.157 mL | 0.3141 mL | 0.6282 mL | 0.7852 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ELN441958
Catalog No.:BCC6452
CAS No.:913064-47-8
- AT13387
Catalog No.:BCC2122
CAS No.:912999-49-6
- Sarafloxacin HCl
Catalog No.:BCC4713
CAS No.:91296-87-6
- Melilotigenin B
Catalog No.:BCN4456
CAS No.:91269-84-0
- SAG
Catalog No.:BCC6390
CAS No.:912545-86-9
- Veliparib dihydrochloride
Catalog No.:BCC2076
CAS No.:912445-05-7
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
- TCB-2
Catalog No.:BCC7421
CAS No.:912342-28-0
- Karavilagenin A
Catalog No.:BCN4455
CAS No.:912329-03-4
- Spantide I
Catalog No.:BCC5808
CAS No.:91224-37-2
- Noscapine HCl
Catalog No.:BCC3819
CAS No.:912-60-7
- H-Met-OtBu.HCl
Catalog No.:BCC2996
CAS No.:91183-71-0
- Almorexant hydrochloride
Catalog No.:BCC5123
CAS No.:913358-93-7
- AMG-458
Catalog No.:BCC3721
CAS No.:913376-83-7
- Brexpiprazole
Catalog No.:BCC4118
CAS No.:913611-97-9
- 1''-Hydroxyerythrinin C
Catalog No.:BCN4066
CAS No.:913690-46-7
- Ropinirole HCl
Catalog No.:BCC4939
CAS No.:91374-20-8
- SC75741
Catalog No.:BCC5448
CAS No.:913822-46-5
- CS 2100
Catalog No.:BCC6221
CAS No.:913827-99-3
- Lu AA 47070
Catalog No.:BCC7977
CAS No.:913842-25-8
- PluriSIn #1 (NSC 14613)
Catalog No.:BCC2305
CAS No.:91396-88-2
- 25-O-ethylcimigenol-3-O-beta-D-xylopyranoside
Catalog No.:BCN1309
CAS No.:914086-57-0
- 9-Nitrocamptothecin
Catalog No.:BCN8448
CAS No.:91421-42-0
- 9-Aminocamptothecin
Catalog No.:BCN2453
CAS No.:91421-43-1
Effects of nicotine in combination with drugs described as positive allosteric nicotinic acetylcholine receptor modulators in vitro: discriminative stimulus and hypothermic effects in mice.[Pubmed:27238974]
Eur J Pharmacol. 2016 Sep 5;786:169-178.
Some drugs that are positive allosteric nAChR modulators in vitro, desformylflustrabromine (dFBr), PNU-120596 and LY 2087101, have not been fully characterized in vivo. These drugs were examined for their capacity to share or modify the hypothermic and discriminative stimulus effects of nicotine (1mg/kg s.c.) in male C57Bl/6J mice. Nicotine, dFBr, and PNU-120596 produced significant hypothermia, whereas LY 2087101 (up to 100mg/kg) did not. Nicotine dose-dependently increased nicotine-appropriate responding and decreased response rate; the respective ED50 values were 0.56mg/kg and 0.91mg/kg. The modulators produced no more than 38% nicotine-appropriate responding up to doses that disrupted operant responding. Rank order potency was the same for hypothermia and rate-decreasing effects: nicotine>dFBr>PNU-120596=LY 2087101. Mecamylamine and the alpha4beta2 nAChR antagonist dihydro-beta-erythroidine, but not the alpha7 antagonist methyllycaconitine, antagonized the hypothermic effects of nicotine. In contrast, mecamylamine did not antagonize the hypothermic effects of the modulators. The combined discriminative stimulus effects of DFBr and nicotine were synergistic, whereas the combined hypothermic effects of nicotine with either dFBr or PNU-120596 were infra-additive. PNU-120596 did not modify the nicotine discriminative stimulus, and LY 2087101 did not significantly modify either effect of nicotine. Positive modulation of nicotine at nAChRs by PNU-120596 and LY 2087101 in vitro does not appear to confer enhancement of the nAChR-mediated hypothermic or discriminative stimulus effects of nicotine. However, dFBr appears to be a positive allosteric modulator of some behavioral effects of nicotine at doses of dFBr smaller than the doses producing unwanted effects (e.g. hypothermia) through non-nAChR mechanisms.
Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators.[Pubmed:16738207]
J Pharmacol Exp Ther. 2006 Sep;318(3):1108-17.
Here we report the discovery, by high-throughput screening, of three novel (2-amino-5-keto)thiazole compounds that act as selective potentiators of nicotinic acetylcholine receptors. Compound selectivity was assessed at seven human nicotinic acetylcholine receptors (alpha1beta1gammadelta, alpha2beta4, alpha3beta2, alpha3beta4, alpha4beta2, alpha4beta4, and alpha7) expressed in mammalian cells or Xenopus oocytes. At alpha2beta4, alpha4beta2, alpha4beta4, and alpha7, but not alpha1beta1gammadelta, alpha3beta2, or alpha3beta4, submaximal responses to nicotinic agonists were potentiated in a concentration-dependent manner by all compounds. At similar concentrations, no potentiation of 5-hydroxytryptamine, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, GABA(A), and N-methyl-d-aspartate receptors or voltage-gated Na(+) and Ca(2+) channels was observed. Furthermore, these compounds did not inhibit acetylcholine esterase. Further profiling revealed that these compounds enhanced the potency and maximal efficacy of a range of nicotinic agonists at alpha4beta2 nicotinic acetylcholine receptors, a profile typical of allosteric potentiators. At concentrations required for potentiation, the compounds did not displace [(3)H]epibatidine from the agonist-binding site, and potentiation was observed at all agonist concentrations, suggesting a noncompetitive mechanism of action. Blockade of common second messenger systems did not affect potentiation. At concentrations higher then required for potentiation the compounds also displayed intrinsic agonist activity, which was blocked by competitive and noncompetitive nicotinic acetylcholine receptor (nAChR) antagonists. These novel selective nicotinic receptor potentiators should help in clarifying the potential therapeutic utility of selective nAChR modulation for the treatment of central nervous system disorders.


