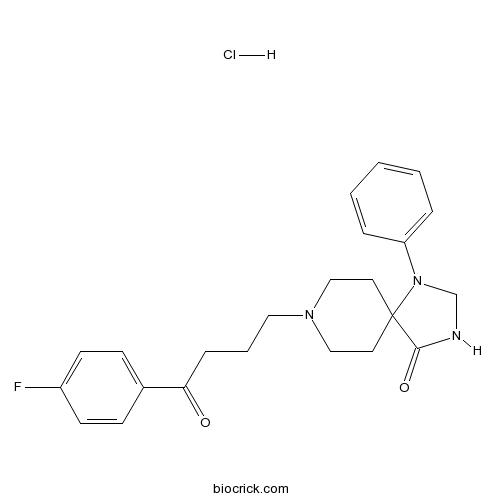
Spiperone hydrochloride5-HT2A antagonist. Also D2-like antagonist CAS# 2022-29-9 |
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- CEP 1347
Catalog No.:BCC7982
CAS No.:156177-65-0
- AEG 3482
Catalog No.:BCC8088
CAS No.:63735-71-7
- AS 602801
Catalog No.:BCC1369
CAS No.:848344-36-5
- CC-930
Catalog No.:BCC1459
CAS No.:899805-25-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 2022-29-9 | SDF | Download SDF |
| PubChem ID | 11957687 | Appearance | Powder |
| Formula | C23H27ClFN3O2 | M.Wt | 431.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | 8-[4-(4-fluorophenyl)-4-oxobutyl]-1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one;hydrochloride | ||
| SMILES | C1CN(CCC12C(=O)NCN2C3=CC=CC=C3)CCCC(=O)C4=CC=C(C=C4)F.Cl | ||
| Standard InChIKey | QUIKMLCZZMOBLH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H26FN3O2.ClH/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20;/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-HT2A serotonin and selective D2-like dopamine receptor antagonist (Ki values are 0.06, 0.6, 0.08, ~ 350, ~ 3500 nM for D2, D3, D4, D1 and D5 receptors respectively). Antipsychotic. |

Spiperone hydrochloride Dilution Calculator

Spiperone hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3151 mL | 11.5757 mL | 23.1514 mL | 46.3027 mL | 57.8784 mL |
| 5 mM | 0.463 mL | 2.3151 mL | 4.6303 mL | 9.2605 mL | 11.5757 mL |
| 10 mM | 0.2315 mL | 1.1576 mL | 2.3151 mL | 4.6303 mL | 5.7878 mL |
| 50 mM | 0.0463 mL | 0.2315 mL | 0.463 mL | 0.9261 mL | 1.1576 mL |
| 100 mM | 0.0232 mL | 0.1158 mL | 0.2315 mL | 0.463 mL | 0.5788 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bilastine
Catalog No.:BCC5263
CAS No.:202189-78-4
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Kaempferol 7-O-rhamnoside
Catalog No.:BCN6489
CAS No.:20196-89-8
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- Epicatechin pentaacetate
Catalog No.:BCN4884
CAS No.:20194-41-6
- PTAC oxalate
Catalog No.:BCC6217
CAS No.:201939-40-4
- Ombuoside
Catalog No.:BCN3711
CAS No.:20188-85-6
- Magnolioside
Catalog No.:BCN2832
CAS No.:20186-29-2
- Pisatin
Catalog No.:BCN3912
CAS No.:20186-22-5
- Tenuifolin
Catalog No.:BCN5005
CAS No.:20183-47-5
- Z-Leu-OH
Catalog No.:BCC2766
CAS No.:2018-66-8
- Ac-Phe-OH
Catalog No.:BCC3005
CAS No.:2018-61-3
- Flucytosine
Catalog No.:BCC3780
CAS No.:2022-85-7
- Spiraeoside
Catalog No.:BCC8251
CAS No.:20229-56-5
- Dregeoside Aa1
Catalog No.:BCN4678
CAS No.:20230-41-5
- Dasycarpol
Catalog No.:BCN7134
CAS No.:202343-57-5
- PNU-159682
Catalog No.:BCC5463
CAS No.:202350-68-3
- Scillascillol
Catalog No.:BCN7008
CAS No.:2023822-39-9
- Scillascillone
Catalog No.:BCN6992
CAS No.:2023822-40-2
- Scillascilloside B-1
Catalog No.:BCN6998
CAS No.:2023822-41-3
- 2,2-Bis(4-chloroformyloxyphenyl)propane
Catalog No.:BCC8491
CAS No.:2024-88-6
- Etoricoxib
Catalog No.:BCC1565
CAS No.:202409-33-4
- Hydroxygenkwanin
Catalog No.:BCN4885
CAS No.:20243-59-8
- 1,3,7-Trihydroxy-2-prenylxanthone
Catalog No.:BCN4886
CAS No.:20245-39-0
Effects of dopaminergic agents on carrageenan hyperalgesia after intrathecal administration to rats.[Pubmed:11334867]
Eur J Pharmacol. 2001 Apr 20;418(1-2):73-7.
The present study explored the role of dopaminergic transmission in spinal cord in a model of carrageenan-induced inflammatory pain by examining the effects of selective agonists and antagonists of dopamine receptors. The results were as follows: (1) trans-(-)-4aR-4,4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g] quinoline hydrochloride (LY171555), a dopamine D(2) receptor agonist, produced anti-hyperalgesia (150 and 300 nmol) or hypoalgesia (300 nmol) in the inflamed hindpaws and non-inflamed hindpaws, respectively; Spiperone hydrochloride (8-[4-(4-fluorophenyl)-4-oxobutyl]-1-phenyl-1,3,8-triazaspiro[4,5]decan-4-one hydrochloride), a dopamine D(2) receptor antagonist, decreased the pain threshold of non-inflamed hindpaws (300 nmol). (2) (+/-)-SKF38393 hydrochloride ((+/-)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrochloride), a dopamine D(1) receptor agonist, had no effect on either hindpaw, even at a higher dose (300 nmol); R(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (R(+)-SCH23390 hydrochloride), a dopamine D(1) receptor antagonist, induced anti-hyperalgesia in the inflamed hindpaws (300 nmol). The present results suggest that the dopaminergic system in the spinal cord is involved in the central modulation of inflammatory hyperalgesia, and that the different effects are probably induced by different receptors.
Effects of dopaminergic agents on carrageenan hyperalgesia in rats.[Pubmed:11011033]
Eur J Pharmacol. 2000 Oct 6;406(1):53-8.
The present study explored the role of central dopaminergic transmission in a model of carrageenan-induced inflammatory pain by examining the effects of selective agonists and antagonists of dopamine receptors. The results were as follow: (1) LY171555 (trans-(-)-4aR-4,4a,5,6,7,8,8a,9-Octahydro-5-propyl-1H-pyrazolo[3, 4-g]quinoline hydrochloride), dopamine D(2) receptor agonist, produced anti-hyperalgesia or hypoalgesia in the inflamed hindpaws and non-inflamed hindpaws, respectively; Spiperone hydrochloride (8-[4-(4-Fluorophenyl)-4-oxobutyl]-1-phenyl-1,3,8-triazaspiro[4, 5]decan-4-one hydrochloride), dopamine D(2) receptor antagonist, decreased the pain threshold of the non-inflamed hindpaws. (2) (+/-)-SKF38393 hydrochloride ((+/-)-1-Phenyl-2,3,4, 5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrochloride), dopamine D(1) receptor agonist, produced anti-hyperalgesia or hypoalgesia when administered in a high dose (600 nmol), and decreased the pain threshold of non-inflamed hindpaws when administered in a low dose (150 nmol); R(+)-SCH23390 hydrochloride (R(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4, 5-tetrahydro-1H-3-benzazepine hydrochloride), dopamine D(1) receptor antagonist, induced anti-hyperalgesia or hypoalgesia, respectively. The present study suggests that the dopaminergic system is involved in the central modulation of inflammatory hyperalgesia, and that the different effects are probably induced by the different receptors.
International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin).[Pubmed:7938165]
Pharmacol Rev. 1994 Jun;46(2):157-203.
It is evident that in the last decade or so, a vast amount of new information has become available concerning the various 5-HT receptor types and their characteristics. This derives from two main research approaches, operational pharmacology, using selective ligands (both agonists and antagonists), and, more recently, molecular biology. Although the scientific community continues to deliberate about the hierarchy of criteria for neurotransmitter receptor characterisation, there seems good agreement between the two approaches regarding 5-HT receptor classification. In addition, the information regarding transduction mechanisms and second messengers is also entirely consistent. Thus, on the basis of these essential criteria for receptor characterisation and classification, there are at least three main groups or classes of 5-HT receptor: 5-HT1, 5-HT2, and 5-HT3. Each group is not only operationally but also structurally distinct, with each receptor group having its own distinct transducing system. The more recently identified 5-HT4 receptor almost undoubtedly represents a fourth 5-HT receptor class on the basis of operational and transductional data, but this will only be definitively shown when the cDNA for the receptor has been cloned and the amino acid sequence of the protein is known. Although those 5-HT receptors that have been fully characterised and classified to date (and, hence, named with confidence) would seem to mediate the majority of the actions of 5-HT throughout the mammalian body, not all receptors for 5-HT are fully encompassed within our scheme of classification. These apparent anomalies must be recognised and need further study. They may or may not represent new groups of 5-HT receptor or subtypes of already known groups of 5-HT receptor. Even though the cDNAs for the 5-ht1E, 5-ht1F, 5-ht5, 5-ht6, and 5-ht7 receptors have been cloned and their amino acid sequence defined, more data are necessary concerning their operational and transductional characteristics before one can be confident of the suitability of their appellations. Therefore, it is important to rationalise in concert all of the available data from studies involving both operational approaches of the classical pharmacological type and those from molecular and cellular biology.(ABSTRACT TRUNCATED AT 400 WORDS)
Dopamine receptor pharmacology.[Pubmed:7940991]
Trends Pharmacol Sci. 1994 Jul;15(7):264-70.
Dopamine receptors are the primary targets in the treatment of schizophrenia, Parkinson's disease, and Huntington's chorea, and are discussed in this review by Philip Seeman and Hubert Van Tol. Improved therapy may be obtained by drugs that selectively target a particular subtype of dopamine receptor. Most antipsychotic drugs block D2 receptors in direct correlation to clinical potency, except clozapine, which prefers D4 receptors. D1 and D2 receptors can enhance each other's actions, possibly through subunits of the G proteins. In schizophrenia, the D2 and D3 receptor density is elevated by 10%, while the D4 receptor density is elevated by 600%. Therefore, D4 receptors may be a target for future antipsychotic drugs. While antipsychotics originally helped to discover dopamine receptors, the five cloned dopamine receptors are now facilitating the discovery of selective antipsychotic and antiparkinson drugs.


