RutamarinCAS# 14882-94-1 |
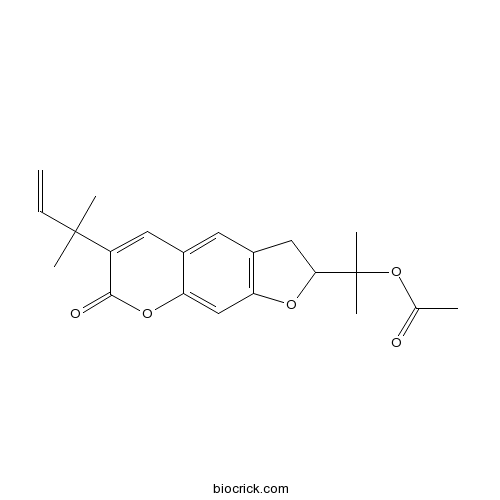
- (+)-Rutamarin
Catalog No.:BCN9204
CAS No.:13164-05-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 14882-94-1 | SDF | Download SDF |
| PubChem ID | 26948 | Appearance | Powder |
| Formula | C21H24O5 | M.Wt | 356.41 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[6-(2-methylbut-3-en-2-yl)-7-oxo-2,3-dihydrofuro[3,2-g]chromen-2-yl]propan-2-yl acetate | ||
| SMILES | CC(=O)OC(C)(C)C1CC2=C(O1)C=C3C(=C2)C=C(C(=O)O3)C(C)(C)C=C | ||
| Standard InChIKey | AWMHMGFGCLBSAY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H24O5/c1-7-20(3,4)15-9-13-8-14-10-18(21(5,6)26-12(2)22)24-16(14)11-17(13)25-19(15)23/h7-9,11,18H,1,10H2,2-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Rutamarin exhibits remarkable cytotoxic activity against HT29 cells (IC50 value of 5.6 uM), it has the potential to be developed as an anticancer agent. 2. (+)-Rutamarin is effective in inhibiting EBV DNA replication and virion production but shows little adverse effect on cell proliferation. 3. (+)-Rutamarin is a dual inducer of both GLUT4 translocation and expression efficiently ameliorates glucose homeostasis in insulin-resistant mice. |
| Targets | Caspase | GLUT | PI3K | Akt |

Rutamarin Dilution Calculator

Rutamarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8058 mL | 14.0288 mL | 28.0576 mL | 56.1151 mL | 70.1439 mL |
| 5 mM | 0.5612 mL | 2.8058 mL | 5.6115 mL | 11.223 mL | 14.0288 mL |
| 10 mM | 0.2806 mL | 1.4029 mL | 2.8058 mL | 5.6115 mL | 7.0144 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bismuth Subsalicylate
Catalog No.:BCC3739
CAS No.:14882-18-9
- Carboxy-PTIO, potassium salt
Catalog No.:BCC6789
CAS No.:148819-94-7
- (R)-2-Methylcysteine HCl
Catalog No.:BCC4017
CAS No.:148766-37-4
- Tyrphostin AG 879
Catalog No.:BCC4514
CAS No.:148741-30-4
- SB 204070
Catalog No.:BCC5752
CAS No.:148688-01-1
- L-733,060 hydrochloride
Catalog No.:BCC5707
CAS No.:148687-76-7
- GR 127935 hydrochloride
Catalog No.:BCC7081
CAS No.:148642-42-6
- Fmoc-Prolinol
Catalog No.:BCC2710
CAS No.:148625-77-8
- 3-O-Methylquercetin
Catalog No.:BCN1660
CAS No.:1486-70-0
- 3-O-Methylquercetin tetraacetate
Catalog No.:BCN1659
CAS No.:1486-69-7
- 3,5-Dihydroxyergosta-7,22-dien-6-one
Catalog No.:BCN1658
CAS No.:14858-07-2
- Pregabalin
Catalog No.:BCN2175
CAS No.:148553-50-8
- Ivabradine HCl
Catalog No.:BCC4350
CAS No.:148849-67-6
- YM 511
Catalog No.:BCC6002
CAS No.:148869-05-0
- HATU
Catalog No.:BCC2813
CAS No.:148893-10-1
- Fmoc-L-Arg(Aloc)2-OH
Catalog No.:BCC2564
CAS No.:148893-34-9
- 3-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl-2-propenal
Catalog No.:BCC8600
CAS No.:148901-68-2
- Fmoc-Inp-OH
Catalog No.:BCC3266
CAS No.:148928-15-8
- Erythritol
Catalog No.:BCN1664
CAS No.:149-32-6
- Scopolamine butylbromide
Catalog No.:BCN5006
CAS No.:149-64-4
- Gallic acid
Catalog No.:BCN1668
CAS No.:149-91-7
- D-Isomenthol
Catalog No.:BCN8540
CAS No.:23283-97-8
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- (S)-WAY 100135 dihydrochloride
Catalog No.:BCC6993
CAS No.:149007-54-5
Rutamarin, an Active Constituent from Ruta angustifolia Pers., Induced Apoptotic Cell Death in the HT29 Colon Adenocarcinoma Cell Line.[Pubmed:28808378]
Pharmacogn Mag. 2017 Jul;13(Suppl 2):S179-S188.
BACKGROUND: Ruta angustifolia Pers. is a perennial herb that is cultivated worldwide, including Southeast Asia, for the treatment of various diseases as traditional medicine. OBJECTIVE: The purpose of the study was to identify an active principle of R. angustifolia and to investigate its effect on the HT29 cell death. MATERIALS AND METHODS: The methanol and fractionated extracts (hexane, chloroform, ethyl acetate, and water) of R. angustifolia Pers. were initially investigated for their cytotoxic activity against two human carcinoma cell lines (MCF7 and HT29) and a normal human colon fibroblast cell line (CCD-18Co) using sulforhodamine B cytotoxicity assay. Eight compounds including Rutamarin were isolated from the active chloroform extract and evaluated for their cytotoxic activity against HT29 human colon carcinoma cell line and CCD-18Co noncancer cells. Further studies on the induction of apoptosis such as morphological examinations, biochemical analyses, cell cycle analysis, and caspase activation assay were conducted in Rutamarin-treated HT29 cells. RESULTS: Rutamarin exhibited remarkable cytotoxic activity against HT29 cells (IC50 value of 5.6 muM) but was not toxic to CCD-18Co cells. The morphological and biochemical hallmarks of apoptosis including activation of caspases 3, 8, and 9 were observed in Rutamarin-treated HT29 cells. These may be associated with cell cycle arrest at the G0/G1 and G2/M checkpoints, which was also observed in HT29 cells. CONCLUSIONS: The present study describes Rutamarin-induced apoptosis in the HT29 cell line for the first time and suggests that Rutamarin has the potential to be developed as an anticancer agent. SUMMARY: Rutamarin was cytotoxic to HT29 colon cancer cells but exerted no damage to normal colon cellsRutamarin induced morphological and biochemical hallmarks of apoptosis in HT29 cellsRutamarin induced cell cycle arrest at the G0/G1 and G2/M checkpoints in a dose-dependent manner in HT29 cellsRutamarin activated caspases 3, 8, and 9 in a dose-dependent manner in HT29 cells. Abbreviations used: ACN: Acetonitrile, ANOVA: One-way analysis of variance, BrdU: Bromodeoxyuridine, 13C-NMR: Carbon-13 Nuclear magnetic resonance, CAD: Caspase-activated endonuclease, CCD-18Co: Human colon normal, DLD1: Human Duke's type C colorectal adenocarcinoma, DMRT: Duncan's multiple range test, DMSO: Dimethyl sulfoxide, DNA: Deoxyribonucleic acid, DR4/5: Death receptor 4/5 protein, EMEM: Eagle's minimum essential media, FBS: Fetal bovine serum, FITC Annexin V: Annexin V conjugated with fluorescein isothiocyanate, FITC-DEVD-FMK: Fluorescein isothiocyanate conjugate of caspase inhibitor Asp-Glu-Val-Asp-fluoromethyl ketone, FITC-IETD-FMK: Fluorescein isothiocyanate conjugate of caspase inhibitor Ile-Glu-Thr-Asp-fluoromethyl ketone, FITC-LEHD-FMK: Fluorescein isothiocyanate conjugate of caspase inhibitor Leu-Glu-His-Asp-fluoromethyl ketone, G0: Quiescent phase of cell cycle, G1: Gap 1 phase of cell cycle, G2: Gap 2 phase of cell cycle, GC-MS: Gas chromatography-mass spectrometry, HeLa: Human cervical adenocarcinoma, HPLC: High performance liquid chromatography, HT29: Human colon adenocarcinoma, Huh7.5: Human hepatocellular carcinoma, IC50: Half maximal inhibitory concentration, KSHV: Kaposi's sarcoma-associated herpesvirus, M phase: Mitotic phase of cell cycle, MCF7: Human breast adenocarcinoma, NMR: Nuclear magnetic resonance, PBS: Phosphate-buffered saline, PI: Propidium iodide, RNase: Ribonuclease, rt: Retention time, S phase: Synthesis phase of cell cycle, SD: Standard deviation, SRB: Sulforhodamine B, TCA: Trichloroacetic acid, TLC: Thin layer chromatography, TNF-R1: Tumor necrosis factor receptor 1 protein, TUNEL: Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling, UV: Ultraviolet.
Antiviral activity of topoisomerase II catalytic inhibitors against Epstein-Barr virus.[Pubmed:24821256]
Antiviral Res. 2014 Jul;107:95-101.
Herpesviruses require several cellular proteins for their lytic DNA replication including topoisomerase II (Topo II). Thus, Topo II could be an effective drug target against herpesviral infection. In this study, we examined several Topo II catalytic inhibitors for their potentials in blocking EBV replication and becoming efficacious antiviral agents. Topo II catalytic inhibitors in general exhibited marked inhibition of EBV lytic replication and minimal cytotoxicity. In particular, (+)-Rutamarin, with the best selectivity index (SI>63) among the inhibitors tested in this study, is effective in inhibiting EBV DNA replication and virion production but shows little adverse effect on cell proliferation, suggesting its potential to become an efficacious and safe drug for the treatment of human diseases associated with EBV infection.
(+)-Rutamarin as a dual inducer of both GLUT4 translocation and expression efficiently ameliorates glucose homeostasis in insulin-resistant mice.[Pubmed:22384078]
PLoS One. 2012;7(2):e31811.
Glucose transporter 4 (GLUT4) is a principal glucose transporter in response to insulin, and impaired translocation or decreased expression of GLUT4 is believed to be one of the major pathological features of type 2 diabetes mellitus (T2DM). Therefore, induction of GLUT4 translocation or/and expression is a promising strategy for anti-T2DM drug discovery. Here we report that the natural product (+)-Rutamarin (Rut) functions as an efficient dual inducer on both insulin-induced GLUT4 translocation and expression. Rut-treated 3T3-L1 adipocytes exhibit efficiently enhanced insulin-induced glucose uptake, while diet-induced obese (DIO) mice based assays further confirm the Rut-induced improvement of glucose homeostasis and insulin sensitivity in vivo. Subsequent investigation of Rut acting targets indicates that as a specific protein tyrosine phosphatase 1B (PTP1B) inhibitor Rut induces basal GLUT4 translocation to some extent and largely enhances insulin-induced GLUT4 translocation through PI3 kinase-AKT/PKB pathway, while as an agonist of retinoid X receptor alpha (RXRalpha), Rut potently increases GLUT4 expression. Furthermore, by using molecular modeling and crystallographic approaches, the possible binding modes of Rut to these two targets have been also determined at atomic levels. All our results have thus highlighted the potential of Rut as both a valuable lead compound for anti-T2DM drug discovery and a promising chemical probe for GLUT4 associated pathways exploration.


