Pseudolaric acid C2CAS# 82508-35-8 |
Quality Control & MSDS
Number of papers citing our products

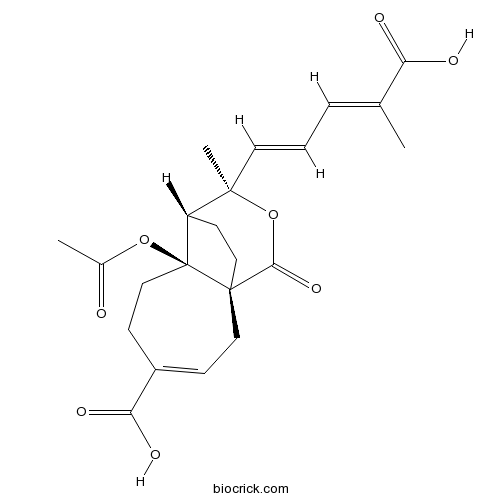
Chemical structure

3D structure
| Cas No. | 82508-35-8 | SDF | Download SDF |
| PubChem ID | 6475945 | Appearance | Powder |
| Formula | C22H26O8 | M.Wt | 418.44 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1~{R},7~{S},8~{S},9~{R})-7-acetyloxy-9-[(1~{E},3~{E})-4-carboxypenta-1,3-dienyl]-9-methyl-11-oxo-10-oxatricyclo[6.3.2.0^{1,7}]tridec-3-ene-4-carboxylic acid | ||
| SMILES | CC(=CC=CC1(C2CCC3(C2(CCC(=CC3)C(=O)O)OC(=O)C)C(=O)O1)C)C(=O)O | ||
| Standard InChIKey | ZPSQWDVEMDWXPJ-HPHAYBORSA-N | ||
| Standard InChI | InChI=1S/C22H26O8/c1-13(17(24)25)5-4-9-20(3)16-8-11-21(19(28)30-20)10-6-15(18(26)27)7-12-22(16,21)29-14(2)23/h4-6,9,16H,7-8,10-12H2,1-3H3,(H,24,25)(H,26,27)/b9-4+,13-5+/t16-,20+,21+,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Metabolic pathway and metabolites of total diterpene acid isolated from Pseudolarix kaempferi.[Pubmed: 25322560]Yao Xue Xue Bao. 2014 Aug;49(8):1169-74.The preliminary metabolic profile of total diterpene acid (TDA) isolated from Pseudolarix kaempferi was investigated by using in vivo and in vitro tests.
|

Pseudolaric acid C2 Dilution Calculator

Pseudolaric acid C2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3898 mL | 11.9491 mL | 23.8983 mL | 47.7966 mL | 59.7457 mL |
| 5 mM | 0.478 mL | 2.3898 mL | 4.7797 mL | 9.5593 mL | 11.9491 mL |
| 10 mM | 0.239 mL | 1.1949 mL | 2.3898 mL | 4.7797 mL | 5.9746 mL |
| 50 mM | 0.0478 mL | 0.239 mL | 0.478 mL | 0.9559 mL | 1.1949 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.239 mL | 0.478 mL | 0.5975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Trikamsteroside C
Catalog No.:BCN8724
CAS No.:952579-35-0
- Oleuroside
Catalog No.:BCN8722
CAS No.:116383-31-4
- Dihydrowithaferin A
Catalog No.:BCN8721
CAS No.:5589-41-3
- 19 alpha-Hydroxyasiatic acid
Catalog No.:BCN8720
CAS No.:70868-78-9
- 3-Methylxanthine
Catalog No.:BCN8719
CAS No.:1076-22-8
- Nagarine
Catalog No.:BCN8718
CAS No.:41849-35-8
- 1-Isomangostin
Catalog No.:BCN8717
CAS No.:19275-44-6
- Indole-3-acetic acid
Catalog No.:BCN8716
CAS No.:87-51-4
- 2-O-Acetyl-20-hydroxyecdysone
Catalog No.:BCN8715
CAS No.:19536-25-5
- Glycinol
Catalog No.:BCN8714
CAS No.:69393-95-9
- Bryonamide A
Catalog No.:BCN8713
CAS No.:75268-14-3
- Methyl caffeate acid
Catalog No.:BCN8712
CAS No.:3843-74-1
- 3-O-Acetyl-20-hydroxyecdysone
Catalog No.:BCN8728
CAS No.:22961-68-8
- N-Benzyloctadecanamide
Catalog No.:BCN8725
CAS No.:5327-45-7
- Hamaudol
Catalog No.:BCN6371
CAS No.:735-46-6
- Celosin I
Catalog No.:BCN8723
CAS No.:1807732-38-2
- Polygalin C
Catalog No.:BCN8738
CAS No.:934768-05-5
- Lucidenic acid F
Catalog No.:BCN6374
CAS No.:98665-18-0
- Irilone
Catalog No.:BCN8188
CAS No.:41653-81-0
- Trikamsteroside D
Catalog No.:BCN8610
CAS No.:952579-36-1
- Podophyllol
Catalog No.:BCN6372
CAS No.:78339-51-2
- Parispseudoside C
Catalog No.:BCN8737
CAS No.:1206707-59-6
- 3'-Hydroxymirificin
Catalog No.:BCN6365
CAS No.:168035-02-7
- Soyasapogenol C
Catalog No.:BCN6388
CAS No.:595-14-2
[Metabolic pathway and metabolites of total diterpene acid isolated from Pseudolarix kaempferi].[Pubmed:25322560]
Yao Xue Xue Bao. 2014 Aug;49(8):1169-74.
The preliminary metabolic profile of total diterpene acid (TDA) isolated from Pseudolarix kaempferi was investigated by using in vivo and in vitro tests. Pseudolaric acid C2 (PC2) was identified as the predominant metabolite in plasma, urine, bile and feces after both oral and intravenous administrations to rats using HPLC-UV and HPLC-ESI/MS(n), and demethoxydeacetoxypseudolaric acid B (DDPB), a metabolite proposed to be the glucoside of PC2 (PC2G), as well as pseudolaric acid C (PC), pseudolaric acid A (PA), pseudolaric acid A O-beta-D glucopyranoside (PAG), pseudolaric acid B O-beta-D glucopyranoside (PBG) and deacetylpseudolaric acid A (DPA) originated from TDA could also be detected. It was demonstrated by tests that the metabolism of TDA is independent of intestinal microflora, and neither of pepsin and trypsin is in charge of metabolism of TDA, TDA is also stable in both pH environments of gastric tract and intestinal tract. The metabolites of TDA in whole blood in vitro incubation were found to be PC2, DDPB and PC2G, which demonstrated that the metabolic reaction of TDA in vivo is mainly occurred in blood and contributed to be the hydrolysis of plasma esterase to ester bond, as well as the glucosylation reaction. These results clarified the metabolic pathway of TDA for the first time, which is of great significance to the in vivo active form and acting mechanism research of P. kaempferi.
[Metabolic pathway and metabolites of pseudolaric acid B].[Pubmed:22260030]
Yao Xue Xue Bao. 2011 Nov;46(11):1361-5.
The metabolic profile of pseudolaric acid B (PB) was investigated by using in vivo and in vitro tests. Pseudolaric acid C2 (PC2) was identified as the specific metabolite of PB in plasma, urine, bile and feces using HPLC and HPLC-ESI/MS(n) after both oral and intravenous administration to rats, and almost no prototype was detected in all kinds of samples. The metabolic behaviors of PB orally administered in rats treated with antibiotics to eliminate intestinal microflora were identical with those in untreated rats, demonstrating that the metabolism of PB is independent of intestinal microflora. PB was stable in 48 h respective incubation with artificial gastric juice and artificial intestinal juice, suggesting that neither pepsin nor trypsin is in charge of metabolism of PB, and also demonstrating that PB is stable in both pH environments of gastric tract and intestinal tract. In vitro research on metabolism of PB in rat liver microsomes incubation revealed that little PB was metabolized and that the proposed metabolites were the demethoxy and demethoxydecarboxy products of the prototype. The amount of metabolites was extremely low compared with the prototype, indicating that liver microsomes are not responsible for the metabolism of PB either. PB was gradually metabolized into PC2 during 1 h in whole blood incubation in vitro, and the metabolic process showed dynamically dependent manner with incubation time. Once absorbed into blood, PB was quickly metabolized into PC2, accordingly, little prototype was detected in all kinds of samples. The metabolism was attributed to the rapid hydrolysis of C-19 ester bond by plasma esterase. These results clarified the metabolic pathway of PB for the first time, which was of great significance to identify the in vivo active form and interpret acting mechanism of the active compounds of P. kaempferi.


