Dihydrowithaferin ACAS# 5589-41-3 |
Quality Control & MSDS
Number of papers citing our products

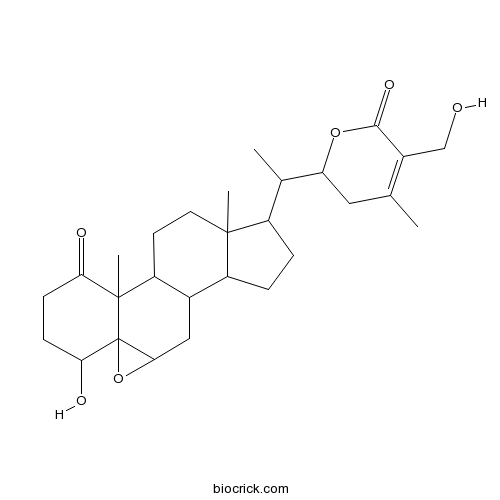
Chemical structure

3D structure
| Cas No. | 5589-41-3 | SDF | Download SDF |
| PubChem ID | 418033 | Appearance | Powder |
| Formula | C28H40O6 | M.Wt | 472.6 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-hydroxy-15-[1-[5-(hydroxymethyl)-4-methyl-6-oxo-2,3-dihydropyran-2-yl]ethyl]-2,16-dimethyl-8-oxapentacyclo[9.7.0.02,7.07,9.012,16]octadecan-3-one | ||
| SMILES | CC1=C(C(=O)OC(C1)C(C)C2CCC3C2(CCC4C3CC5C6(C4(C(=O)CCC6O)C)O5)C)CO | ||
| Standard InChIKey | YRXCLNDPESBJHL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H40O6/c1-14-11-21(33-25(32)17(14)13-29)15(2)18-5-6-19-16-12-24-28(34-24)23(31)8-7-22(30)27(28,4)20(16)9-10-26(18,19)3/h15-16,18-21,23-24,29,31H,5-13H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 2, 3-Dihydrowithaferin A in the diet may prevent or decrease the growth of tumors in human. |
| In vitro | Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves.[Pubmed: 14575818]Life Sci. 2003 Nov 21;74(1):125-32.Ayurvedic medicines prepared in India consist of Withania somnifera roots as one of the main ingredients. It is consumed as a dietary supplement around the world. The leaves of W. somnifera were used in the treatment of tumors and inflammation in several Asian countries.
|

Dihydrowithaferin A Dilution Calculator

Dihydrowithaferin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.116 mL | 10.5798 mL | 21.1595 mL | 42.3191 mL | 52.8989 mL |
| 5 mM | 0.4232 mL | 2.116 mL | 4.2319 mL | 8.4638 mL | 10.5798 mL |
| 10 mM | 0.2116 mL | 1.058 mL | 2.116 mL | 4.2319 mL | 5.2899 mL |
| 50 mM | 0.0423 mL | 0.2116 mL | 0.4232 mL | 0.8464 mL | 1.058 mL |
| 100 mM | 0.0212 mL | 0.1058 mL | 0.2116 mL | 0.4232 mL | 0.529 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 19 alpha-Hydroxyasiatic acid
Catalog No.:BCN8720
CAS No.:70868-78-9
- 3-Methylxanthine
Catalog No.:BCN8719
CAS No.:1076-22-8
- Nagarine
Catalog No.:BCN8718
CAS No.:41849-35-8
- 1-Isomangostin
Catalog No.:BCN8717
CAS No.:19275-44-6
- Indole-3-acetic acid
Catalog No.:BCN8716
CAS No.:87-51-4
- 2-O-Acetyl-20-hydroxyecdysone
Catalog No.:BCN8715
CAS No.:19536-25-5
- Glycinol
Catalog No.:BCN8714
CAS No.:69393-95-9
- Bryonamide A
Catalog No.:BCN8713
CAS No.:75268-14-3
- Methyl caffeate acid
Catalog No.:BCN8712
CAS No.:3843-74-1
- Quercetin 3-O-beta-(6''-p-coumaroyl)glucopyranosyl(1->2)-alpha-L-rhamnopyranoside
Catalog No.:BCN8711
CAS No.:143061-65-8
- Asiaticoside B
Catalog No.:BCN8709
CAS No.:125265-68-1
- Isocarlinoside
Catalog No.:BCN8708
CAS No.:83151-90-0
- Oleuroside
Catalog No.:BCN8722
CAS No.:116383-31-4
- Trikamsteroside C
Catalog No.:BCN8724
CAS No.:952579-35-0
- Pseudolaric acid C2
Catalog No.:BCN8726
CAS No.:82508-35-8
- 3-O-Acetyl-20-hydroxyecdysone
Catalog No.:BCN8728
CAS No.:22961-68-8
- N-Benzyloctadecanamide
Catalog No.:BCN8725
CAS No.:5327-45-7
- Hamaudol
Catalog No.:BCN6371
CAS No.:735-46-6
- Celosin I
Catalog No.:BCN8723
CAS No.:1807732-38-2
- Polygalin C
Catalog No.:BCN8738
CAS No.:934768-05-5
- Lucidenic acid F
Catalog No.:BCN6374
CAS No.:98665-18-0
- Irilone
Catalog No.:BCN8188
CAS No.:41653-81-0
- Trikamsteroside D
Catalog No.:BCN8610
CAS No.:952579-36-1
- Podophyllol
Catalog No.:BCN6372
CAS No.:78339-51-2
2,3-Dihydrowithaferin A-3beta-O-sulfate, a new potential prodrug of withaferin A from aeroponically grown Withania somnifera.[Pubmed:19056281]
Bioorg Med Chem. 2009 Mar 15;17(6):2210-4.
Preparations of the roots of the medicinal plant Withania somnifera (L.) Dunal commonly called ashwagandha have been used for millennia in the Ayurvedic medical tradition of India as a general tonic to relieve stress and enhance health, especially in the elderly. In modern times, ashwagandha has been shown to possess intriguing antiangiogenic and anticancer activity, largely attributable to the presence of the steroidal lactone withaferin A as the major constituent. When cultured using the aeroponic technique, however, this plant was found to produce a new natural product, 2,3-Dihydrowithaferin A-3beta-O-sulfate (1), as the predominant constituent of methanolic extracts prepared from aerial tissues. The characteristic bioactivities exhibited by 1 including inhibition of cancer cell proliferation/survival, disruption of cytoskeletal organization and induction of the cellular heat-shock response paralleled those displayed by withaferin A (2). The delayed onset of action and reduced potency of 1 in cell culture along with previous observations demonstrating the requirement of the 2(3)-double bond in withanolides for bioactivity suggested that 1 might be converted to 2 in cell culture media and this was confirmed by HPLC analysis. The abundant yield of 1 from aeroponically cultivated plants, its good aqueous solubility and spontaneous conversion to 2 under cell culture conditions, suggest that 1 could prove useful as a readily formulated prodrug of withaferin A that merits further evaluation in animal models.


