PinostilbeneCAS# 42438-89-1 |
Quality Control & MSDS
Number of papers citing our products

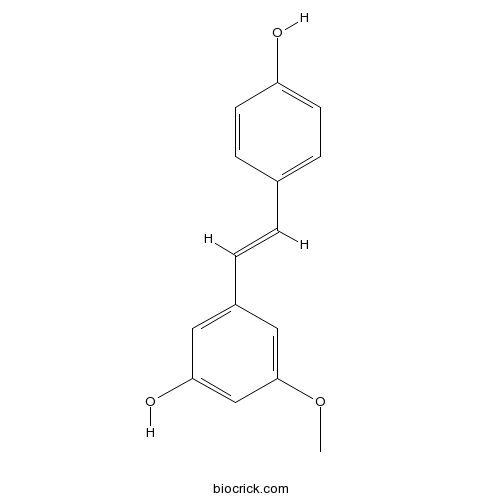
Chemical structure

3D structure
| Cas No. | 42438-89-1 | SDF | Download SDF |
| PubChem ID | 5473050 | Appearance | White-beige powder |
| Formula | C15H14O3 | M.Wt | 242.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | 3,4'-Dihydroxy 5-methoxy trans-stilbene | ||
| Solubility | Soluble in methan | ||
| Chemical Name | 3-[(E)-2-(4-hydroxyphenyl)ethenyl]-5-methoxyphenol | ||
| SMILES | COC1=CC(=CC(=C1)O)C=CC2=CC=C(C=C2)O | ||
| Standard InChIKey | KUWZXOMQXYWKBS-NSCUHMNNSA-N | ||
| Standard InChI | InChI=1S/C15H14O3/c1-18-15-9-12(8-14(17)10-15)3-2-11-4-6-13(16)7-5-11/h2-10,16-17H,1H3/b3-2+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pinostilbene has protective effects against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells, it can reduce release of lactate dehydrogenase and activity of caspase-3 triggered by 6-hydroxydopamine (6-OHDA) in a dose-dependent manner. 2. Pinostilbene can significantly inhibit the growth of human colon cancer cells, i.e., HCT116 and HT29, 20 and 40 uM of pinostilbene causes cell cycle arrest at S phase and induces apoptosis in colon cancer cells. |
| Targets | Caspase | JNK |

Pinostilbene Dilution Calculator

Pinostilbene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1271 mL | 20.6356 mL | 41.2712 mL | 82.5423 mL | 103.1779 mL |
| 5 mM | 0.8254 mL | 4.1271 mL | 8.2542 mL | 16.5085 mL | 20.6356 mL |
| 10 mM | 0.4127 mL | 2.0636 mL | 4.1271 mL | 8.2542 mL | 10.3178 mL |
| 50 mM | 0.0825 mL | 0.4127 mL | 0.8254 mL | 1.6508 mL | 2.0636 mL |
| 100 mM | 0.0413 mL | 0.2064 mL | 0.4127 mL | 0.8254 mL | 1.0318 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pashanone
Catalog No.:BCN5482
CAS No.:42438-78-8
- 1-Benzylimidazole
Catalog No.:BCC8462
CAS No.:4238-71-5
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
- Buxtamine
Catalog No.:BCC8135
CAS No.:4236-73-1
- (-)-Gallocatechin gallate
Catalog No.:BCN6328
CAS No.:4233-96-9
- SC 57461A
Catalog No.:BCC2348
CAS No.:423169-68-0
- PYZD-4409
Catalog No.:BCC4253
CAS No.:423148-78-1
- N-(4-Cyanophenyl)glycine
Catalog No.:BCC9057
CAS No.:42288-26-6
- Hesperadin
Catalog No.:BCC2174
CAS No.:422513-13-1
- Triacetyl Resveratrol
Catalog No.:BCC6482
CAS No.:42206-94-0
- DOI hydrochloride
Catalog No.:BCC5925
CAS No.:42203-78-1
- Cephalocyclidin A
Catalog No.:BCN5481
CAS No.:421583-14-4
- H-ß-Ala-OEt.HCl
Catalog No.:BCC2852
CAS No.:4244-84-2
- Flunixin Meglumin
Catalog No.:BCC4429
CAS No.:42461-84-7
- Isobutyl 4-Hydroxybenzoate
Catalog No.:BCN8412
CAS No.:4247-02-3
- 23-Hydroxylongispinogenin
Catalog No.:BCN7830
CAS No.:42483-24-9
- IPA-3
Catalog No.:BCC4978
CAS No.:42521-82-4
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Crocin
Catalog No.:BCN2373
CAS No.:42553-65-1
- Sotrastaurin (AEB071)
Catalog No.:BCC3857
CAS No.:425637-18-9
- 8-Hydroxy-5-O-beta-D-glucopyranosylpsoralen
Catalog No.:BCN1443
CAS No.:425680-98-4
- Luteolin-6-C-glucoside
Catalog No.:BCN4985
CAS No.:4261-42-1
- TAK-700 (Orteronel)
Catalog No.:BCC2280
CAS No.:426219-18-3
- TAK-700 salt
Catalog No.:BCC1979
CAS No.:426219-53-6
Protective effects of pinostilbene, a resveratrol methylated derivative, against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells.[Pubmed:19443200]
J Nutr Biochem. 2010 Jun;21(6):482-9.
Resveratrol (3,4',5-trans-trihydroxystilbene) is a phytoalexin with emerging lines of evidence supporting its beneficial effects on cardiovascular systems and inhibition of carcinogenesis. It has also been reported that certain methylated resveratrol derivatives are more effective than resveratrol in the prevention/treatment of cancer. However, little is known about the impact of resveratrol and its derivatives on the development of Parkinson's disease. In this study, we compared the neuroprotective effects of resveratrol with four methylated (fully or partially) resveratrol derivatives against parkinsonian mimetic 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in SH-SY5Y cells. Release of lactate dehydrogenase and activity of caspase-3 triggered by 6-OHDA were significantly reduced by resveratrol and one of the methylated derivatives, Pinostilbene (3,4'-dihydroxy-5-methoxystilbene), in a dose-dependent manner. In addition, Pinostilbene exerted a potent neuroprotective effect with a wider effective concentration range than resveratrol. By using high-performance liquid chromatography, we found that uptake of Pinostilbene into SH-SY5Y cells was significantly higher than that of resveratrol. Enhanced bioavailability may thus be a major factor contributing to the neuroprotective activity of Pinostilbene. Moreover, Western blot analysis demonstrated that Pinostilbene markedly attenuated the phosphorylation of JNK and c-Jun triggered by 6-OHDA. Besides, mammalian target of rapamycin kinase may be an intracellular target accounting for the neuroprotective effects of Pinostilbene. Our findings demonstrate the potential of methylated stilbenes in neuroprotection and provide important information for further research in this field.


