23-HydroxylongispinogeninCAS# 42483-24-9 |
Quality Control & MSDS
Number of papers citing our products

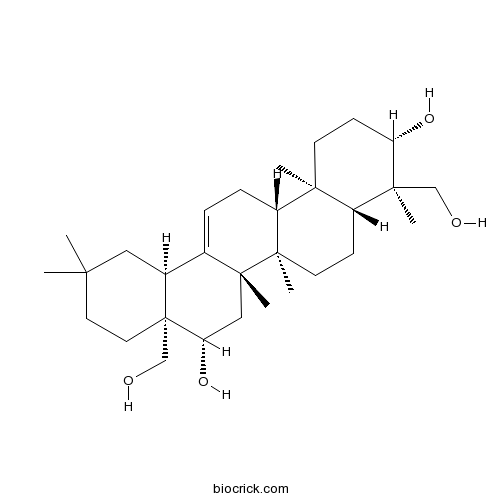
Chemical structure

3D structure
| Cas No. | 42483-24-9 | SDF | Download SDF |
| PubChem ID | 13322806 | Appearance | Powder |
| Formula | C30H50O4 | M.Wt | 474.72 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,4R,4aR,6aR,6bS,8S,8aS,12aS,14aR,14bR)-4,8a-bis(hydroxymethyl)-4,6a,6b,11,11,14b-hexamethyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicene-3,8-diol | ||
| SMILES | CC1(CCC2(C(C1)C3=CCC4C5(CCC(C(C5CCC4(C3(CC2O)C)C)(C)CO)O)C)CO)C | ||
| Standard InChIKey | IACGAAXNDKIGSX-FMGQVEPTSA-N | ||
| Standard InChI | InChI=1S/C30H50O4/c1-25(2)13-14-30(18-32)20(15-25)19-7-8-22-26(3)11-10-23(33)27(4,17-31)21(26)9-12-28(22,5)29(19,6)16-24(30)34/h7,20-24,31-34H,8-18H2,1-6H3/t20-,21+,22+,23-,24-,26-,27-,28+,29+,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Journal of the Chemical Society, Perkin Transactions 1, 1987.Triterpenoid glycosides of Corchorus acutangulus Lam.[Reference: WebLink]
|

23-Hydroxylongispinogenin Dilution Calculator

23-Hydroxylongispinogenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1065 mL | 10.5325 mL | 21.065 mL | 42.1301 mL | 52.6626 mL |
| 5 mM | 0.4213 mL | 2.1065 mL | 4.213 mL | 8.426 mL | 10.5325 mL |
| 10 mM | 0.2107 mL | 1.0533 mL | 2.1065 mL | 4.213 mL | 5.2663 mL |
| 50 mM | 0.0421 mL | 0.2107 mL | 0.4213 mL | 0.8426 mL | 1.0533 mL |
| 100 mM | 0.0211 mL | 0.1053 mL | 0.2107 mL | 0.4213 mL | 0.5266 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isobutyl 4-Hydroxybenzoate
Catalog No.:BCN8412
CAS No.:4247-02-3
- Flunixin Meglumin
Catalog No.:BCC4429
CAS No.:42461-84-7
- H-ß-Ala-OEt.HCl
Catalog No.:BCC2852
CAS No.:4244-84-2
- Pinostilbene
Catalog No.:BCN5483
CAS No.:42438-89-1
- Pashanone
Catalog No.:BCN5482
CAS No.:42438-78-8
- 1-Benzylimidazole
Catalog No.:BCC8462
CAS No.:4238-71-5
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
- Buxtamine
Catalog No.:BCC8135
CAS No.:4236-73-1
- (-)-Gallocatechin gallate
Catalog No.:BCN6328
CAS No.:4233-96-9
- SC 57461A
Catalog No.:BCC2348
CAS No.:423169-68-0
- PYZD-4409
Catalog No.:BCC4253
CAS No.:423148-78-1
- N-(4-Cyanophenyl)glycine
Catalog No.:BCC9057
CAS No.:42288-26-6
- IPA-3
Catalog No.:BCC4978
CAS No.:42521-82-4
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Crocin
Catalog No.:BCN2373
CAS No.:42553-65-1
- Sotrastaurin (AEB071)
Catalog No.:BCC3857
CAS No.:425637-18-9
- 8-Hydroxy-5-O-beta-D-glucopyranosylpsoralen
Catalog No.:BCN1443
CAS No.:425680-98-4
- Luteolin-6-C-glucoside
Catalog No.:BCN4985
CAS No.:4261-42-1
- TAK-700 (Orteronel)
Catalog No.:BCC2280
CAS No.:426219-18-3
- TAK-700 salt
Catalog No.:BCC1979
CAS No.:426219-53-6
- 21,23-Dihydro-23-hydroxy-21-oxozapoterin
Catalog No.:BCN7230
CAS No.:426266-88-8
- Dehydrodiconiferyl alcohol
Catalog No.:BCN6878
CAS No.:4263-87-0
- Hemapolin
Catalog No.:BCC8994
CAS No.:4267-80-5
- 4-Hydroxy-3-methoxyphenyl O-beta-D-6-O-syringate-glucopyranoside
Catalog No.:BCN1442
CAS No.:426821-85-4
Titerpenoid saponins from Atriplex semibaccata.[Pubmed:12939032]
Z Naturforsch C. 2003 Jul-Aug;58(7-8):485-9.
Four new triterpenoid saponins, 3-O-([beta-D-glucopyranosyl-(1-->2)]-beta-D-galactopyranosyl)-11alpha-methoxy-23- hydroxylongispinogenin, 3-O-([beta-D-glucopyranosyl-(1-->2)]-beta-D-galactopyranosyl)-11alpha-methoxy-23, 29-dihydroxylongispinogenin, 3-O-([beta-D-glucopyranosyl-(1-->2)]-beta-D-galactopyranosyl)-29-hydroxysaikogeni n F and 3-O-([beta-D-glucopyranosyl-(1-->2)]-beta-D-galactopyranosyl)-saikogenin F, have been isolated from Atriplex semibaccata. The structures were determined primarily by NMR spectroscopy. The assignment of NMR signals was performed by means of 1H-1H COSY, ROESY, HMQC and HMBC experiments.
Medicinal foodstuffs. X. Structures of new triterpene glycosides, gymnemosides-c, -d, -e, and -f, from the leaves of Gymnema sylvestre R. Br.: influence of gymnema glycosides on glucose uptake in rat small intestinal fragments.[Pubmed:9433774]
Chem Pharm Bull (Tokyo). 1997 Dec;45(12):2034-8.
Following the characterization of gymnemosides-a and -b, new triterpene glycosides, gymnemosides-c, -d, -e, and -f, were isolated from the leaves of Gymnema (G.) sylvestre R. BR. Their chemical structures were elucidated on the basis of chemical and physicochemical evidence as follows: 21-O-benzoyl-28-O-acetylgymnemagenin 3-O-beta-D-glucopyranosiduronic acid (gymnemoside-c), 23-O-[beta-D-xylopyranosyl (1-->6)-beta-D-glucopyranosyl (1-->6)-beta-D-glucopyranosyl] gymnestrogenin (gymnemoside-d), 23-O-[beta-D-xylopyranosyl (1-->6)-beta-D-glucopyranosyl (1-->6)-beta-D- glucopyranosyl]-28-O-[beta-D-glucopyranosyl (1-->6)-beta-D-glucopyranosyl] 23-Hydroxylongispinogenin (gymnemoside-e), 23-O-[beta-D-xylopyranosyl (1-->6)-beta-D-glucopyranosyl (1-->6)-beta-D-glucopyranosyl]-28-O-[beta-O-glucopyranosyl (1-->6)-beta-D-glucopyranosyl] 3 beta,16 beta,23,28-tetrahydroxyolean-18-ene (gymnemoside-f). The inhibitory effects of gymnemosides-c, -d, -e, and -f and principal triterpene glycosides from G. sylvestre on glucose uptake in rat small intestinal fragments were examined, and gymnemic acids II, III, and IV, gymnemasaponin V, and gymnemoside-f were found to exhibit the inhibitory activity.
Two antiproliferative saponins of Tarenna grevei from the Madagascar dry forest [1].[Pubmed:22816288]
Nat Prod Commun. 2012 Jun;7(6):705-8.
Antiproliferative bioassay-guided fractionation of the ethanol extract of the endemic Malagasy Rubiaceous plant Tarenna grevei led to the isolation of two new antiproliferative oxygenated oleanane triterpene saponins. The structures of the two active compounds were elucidated as 23-Hydroxylongispinogenin 3-O-beta-D-glucopyranoside (1) and longispinogenin 3-O-beta-D-glucopyranosyl (1 --> 2)-beta-D-glucopyranoside (3) by analyses of their spectral data including 1D- and 2D-NMR spectroscopy and chemical evidence. Compounds 1 and 3 displayed moderate antiproliferative activity against the A2780 ovarian cancer cell line with IC50 values of 7.6 and 4 microM, respectively.


