Mesulergine hydrochloride5-HT2A and 5-HT2C antagonist. Also dopamine receptor partial agonist CAS# 72786-12-0 |
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Cefoselis Sulfate
Catalog No.:BCC4769
CAS No.:122841-12-7
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 72786-12-0 | SDF | Download SDF |
| PubChem ID | 155746 | Appearance | Powder |
| Formula | C18H27ClN4O2S | M.Wt | 398.95 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CU-32-085 | ||
| Solubility | Soluble to 100 mM in DMSO and to 5 mM in water with gentle warming | ||
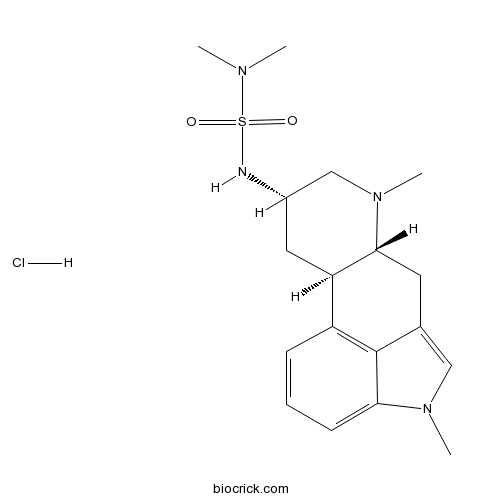
| Chemical Name | (6aR,9S,10aR)-9-(dimethylsulfamoylamino)-4,7-dimethyl-6,6a,8,9,10,10a-hexahydroindolo[4,3-fg]quinoline;hydrochloride | ||
| SMILES | CN1CC(CC2C1CC3=CN(C4=CC=CC2=C34)C)NS(=O)(=O)N(C)C.Cl | ||
| Standard InChIKey | HANSYUJEPWNHIM-IVMONYBCSA-N | ||
| Standard InChI | InChI=1S/C18H26N4O2S.ClH/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13;/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3;1H/t13-,15+,17+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-HT2A and 2C receptor antagonist (pA2 values are 9.1 for each receptor) and D2-like dopamine receptor partial agonist (Ki = 8 nM). Displays antiprolactin and antiparkinsonian effects in vivo. |

Mesulergine hydrochloride Dilution Calculator

Mesulergine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5066 mL | 12.5329 mL | 25.0658 mL | 50.1316 mL | 62.6645 mL |
| 5 mM | 0.5013 mL | 2.5066 mL | 5.0132 mL | 10.0263 mL | 12.5329 mL |
| 10 mM | 0.2507 mL | 1.2533 mL | 2.5066 mL | 5.0132 mL | 6.2664 mL |
| 50 mM | 0.0501 mL | 0.2507 mL | 0.5013 mL | 1.0026 mL | 1.2533 mL |
| 100 mM | 0.0251 mL | 0.1253 mL | 0.2507 mL | 0.5013 mL | 0.6266 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- β-Funaltrexamine hydrochloride
Catalog No.:BCC6850
CAS No.:72786-10-8
- Senecivernine
Catalog No.:BCN2135
CAS No.:72755-25-0
- Odorinol
Catalog No.:BCN4284
CAS No.:72755-22-7
- Odorine
Catalog No.:BCN4283
CAS No.:72755-20-5
- 1-Ethoxycarbonyl-beta-carboline
Catalog No.:BCN3102
CAS No.:72755-19-2
- Swainsonine
Catalog No.:BCC7602
CAS No.:72741-87-8
- H-D-Lys-OH.HCl
Catalog No.:BCC2989
CAS No.:7274-88-6
- Ilicol
Catalog No.:BCN4282
CAS No.:72715-02-7
- Mollisorin A
Catalog No.:BCN7236
CAS No.:72704-04-2
- Prosapogenin CP6
Catalog No.:BCN2535
CAS No.:72629-76-6
- γ1-MSH
Catalog No.:BCC6021
CAS No.:72629-65-3
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- ICI 118,551 hydrochloride
Catalog No.:BCC4029
CAS No.:72795-01-8
- Linderalactone
Catalog No.:BCN1251
CAS No.:728-61-0
- Estropipate
Catalog No.:BCC7719
CAS No.:7280-37-7
- Panaxydol
Catalog No.:BCN3701
CAS No.:72800-72-7
- OSI-930
Catalog No.:BCC1253
CAS No.:728033-96-3
- Atanine
Catalog No.:BCN3317
CAS No.:7282-19-1
- Deoxyartemisinin
Catalog No.:BCN4285
CAS No.:72826-63-2
- MIRA-1
Catalog No.:BCC2409
CAS No.:72835-26-8
- 1-Benzyl-5-phenylbarbituric acid
Catalog No.:BCC8461
CAS No.:72846-00-5
- Echitoveniline
Catalog No.:BCN7490
CAS No.:72855-79-9
- Crocandine
Catalog No.:BCN2070
CAS No.:72855-83-5
- Agatharesinol
Catalog No.:BCN4594
CAS No.:7288-11-1
Animal model of posthypoxic myoclonus: effects of serotonergic antagonists.[Pubmed:9921842]
Neurology. 1999 Jan 1;52(1):16-21.
OBJECTIVE: To study specific serotonin (5-hydroxytryptamine [5-HT]) receptor subtype antagonists in an animal model of posthypoxic myoclonus. BACKGROUND: Although serotonergic system dysfunction is implicated in posthypoxic myoclonus, anatomic specificity and linkage to receptor subtypes are not delineated. METHODS: The authors performed a pharmacologic study to identify specific serotonin receptor subtype antagonists effective in inhibiting myoclonus in posthypoxic rats. Sprague-Dawley rats underwent cardiac arrest for 8 minutes and were resuscitated. On the day of pharmacologic testing, animals were rated every 10 minutes at -30 minutes to time 0 (drug injection) and from +60 to +150 minutes. Using a blinded methodology, animals were injected with normal saline, vehicle, or one of seven serotonin antagonists given at a dose that maintains serotonin receptor subtype specificity: WAY100135 (5-HT1A), methiothepin mesylate (5-HT1B/1D/2), Mesulergine hydrochloride (5-HT2A/2B), GR 127935 (5-HT1D), SR 46349 (5-HT2), ondansetron (5-HT3), or GR 125487 (5-HT4). Drugs that produced a significant decrease in myoclonus compared with the control were studied in a dose-response study with six doses across a range from the original dose studied to 10% of that dose. RESULTS: Two drugs were significantly different from placebo: methiothepin mesylate and Mesulergine hydrochloride. GR 127935 showed a trend toward reducing myoclonus. Dose-response studies showed that all doses of methiothepin mesylate and the three highest doses of Mesulergine hydrochloride inhibited myoclonus effectively. CONCLUSIONS: 5-HT1B, 5-HT2A/2B, and possibly 5-HT1D receptor subtypes likely play a role in posthypoxic myoclonus. More specific 5-HT antagonists that affect these receptor subtypes are candidates for future testing in this model and in Lance-Adams syndrome.
5-Hydroxytryptophan-induced myoclonus in guinea pigs: mediation through 5-HT1/2 receptor subtypes.[Pubmed:9650847]
Eur J Pharmacol. 1998 Apr 17;347(1):51-6.
In guinea pigs, myoclonus can be induced by 5-hydroxytryptamine (5-HT, serotonin) precursors and synthetic 5-HT receptor agonists, yet the receptor subtype specificity of this behavior is not fully delineated. Guinea pigs were pre-treated with carbidopa (50 mg) followed by one of eight 5-HT antagonists: (-)-N-tert-butyl-3-[4-(2-methoxyphenyl) piperazin-1-yl]-2-phenyl propionamide ((-)-WAY 100135) (5-HT1A), N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]-ethyl]-N-(2-pyridyl)-cy clohexancarboxamide (WAY 100635) (5-HT1A), methiothepin mesylate (5-HT1/2), Mesulergine hydrochloride (5-HT2A/2C), N[4-methoxy-3-(4-methyl-L-piperazinyl)phenyl]-2'-methyl-4'-(5-methyl-1,2 ,4-oxadizol-3-yl) (GR 127935) (5-HT1D), trans-4-[(3Z)3-(2-dimethylaminoethyl)oxyimino-3(2-fluorop hen yl) propen-1-yl]phenol, hemifumarate (SR 46349) (5-HT2), ondansetron hydrochloride (5-HT3), and [1-[2-[methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl-5-fluoro-2-meth oxy-1H-indole-3-carboxylate (GR 125487) (5-HT4). Thirty minutes later, they received 5-hydroxytryptophan (5-HTP) (75 mg/kg, sc) and myoclonic jumping rates were assessed every 10 min for 200 min by a blinded observer. Repeated measures analysis of variance of drug-induced antagonism of 5-HTP-induced myoclonus revealed a significant effect for the 5-HT receptor antagonists methiothepin mesylate, GR127935, and Mesulergine hydrochloride compared to placebo, and each of these drugs inhibited 5-HTP-induced myoclonus in a dose-dependent fashion. Based on the receptor profiles of the three effective antagonists, 5-HTP-induced myoclonus is influenced by the 5-HT1/2 receptor systems. The absence of a significant change with any other receptor subtype antagonist suggests that myoclonus is not related to diffuse activation of central serotonergic mechanisms.
International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin).[Pubmed:7938165]
Pharmacol Rev. 1994 Jun;46(2):157-203.
It is evident that in the last decade or so, a vast amount of new information has become available concerning the various 5-HT receptor types and their characteristics. This derives from two main research approaches, operational pharmacology, using selective ligands (both agonists and antagonists), and, more recently, molecular biology. Although the scientific community continues to deliberate about the hierarchy of criteria for neurotransmitter receptor characterisation, there seems good agreement between the two approaches regarding 5-HT receptor classification. In addition, the information regarding transduction mechanisms and second messengers is also entirely consistent. Thus, on the basis of these essential criteria for receptor characterisation and classification, there are at least three main groups or classes of 5-HT receptor: 5-HT1, 5-HT2, and 5-HT3. Each group is not only operationally but also structurally distinct, with each receptor group having its own distinct transducing system. The more recently identified 5-HT4 receptor almost undoubtedly represents a fourth 5-HT receptor class on the basis of operational and transductional data, but this will only be definitively shown when the cDNA for the receptor has been cloned and the amino acid sequence of the protein is known. Although those 5-HT receptors that have been fully characterised and classified to date (and, hence, named with confidence) would seem to mediate the majority of the actions of 5-HT throughout the mammalian body, not all receptors for 5-HT are fully encompassed within our scheme of classification. These apparent anomalies must be recognised and need further study. They may or may not represent new groups of 5-HT receptor or subtypes of already known groups of 5-HT receptor. Even though the cDNAs for the 5-ht1E, 5-ht1F, 5-ht5, 5-ht6, and 5-ht7 receptors have been cloned and their amino acid sequence defined, more data are necessary concerning their operational and transductional characteristics before one can be confident of the suitability of their appellations. Therefore, it is important to rationalise in concert all of the available data from studies involving both operational approaches of the classical pharmacological type and those from molecular and cellular biology.(ABSTRACT TRUNCATED AT 400 WORDS)
Dopamine agonistic potency of two novel prolactin release-inhibiting ergolines.[Pubmed:6468500]
Eur J Pharmacol. 1984 Jun 1;101(3-4):263-6.
Two novel 8 alpha-amino ergolines (CH 29-717 and CU 32-085) have been shown to inhibit secretion of prolactin in rats in vivo. However, when tested for dopaminomimetic potency on pituitary cell culture preparations in vitro, CH 29-717 inhibited prolactin release with an IC50 = 4 X 10(-9) M. In the same model the 1-methyl-derivative CU 32-085 was only weakly active even at high concentrations. When both compounds were incubated together, CU 32-085 displayed an inhibitory effect on the prolactin release restriction caused by CH 29-717. The activities were not time-dependent since similar results were obtained with incubation for 3 or 24 h. These results support the hypothesis that the 1-methyl ergoline derivative CU 32-085 is a partial agonist and becomes fully active in vivo only after metabolic conversion.
Mesulergine and its 1,20-N,N-bidemethylated metabolite interact directly with D1- and D2-receptors.[Pubmed:6230246]
Eur J Pharmacol. 1983 Nov 11;95(1-2):101-7.
Mesulergine (CU 32-085), an 8 alpha-aminoergoline, has been reported to influence striatal dopamine turnover in a time-dependent biphasic manner, suggesting initial dopamine antagonistic and late dopamine agonistic effects. To clarify whether these opposing in vivo effects are due to a metabolic conversion in vivo or reflect mixed antagonist/agonist effects expressed at different dose levels, mesulergine and a 1,20-N,N-bidemethylated metabolite, identified in rat urine, were investigated in functional dopamine receptor models. Dopamine-sensitive adenylate cyclase in homogenates of rat striatum and modulation of electrically evoked tritium overflow from rat striatal slices previously labelled with [3H]choline were used as tests for D1- and D2-receptors, respectively. Mesulergine was found to antagonise D1-receptor responses at micromolar, and D2-receptor responses at nanomolar concentrations. In contrast, the bidemethylated metabolite of mesulergine stimulated both D1- and D2-receptors at micromolar and nanomolar concentrations, respectively. These in vitro results suggest that at dopamine receptors, mesulergine has antagonistic effects and that the late agonistic effects seen in vivo are mostly due to metabolic conversion into a potent dopaminomimetic drug.


