Meropenem trihydrateBroad-spectum β-lactam antibiotic CAS# 119478-56-7 |
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Cefoselis Sulfate
Catalog No.:BCC4769
CAS No.:122841-12-7
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 119478-56-7 | SDF | Download SDF |
| PubChem ID | 60706 | Appearance | Powder |
| Formula | C17H31N3O8S | M.Wt | 437.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (228.57 mM; Need ultrasonic) | ||
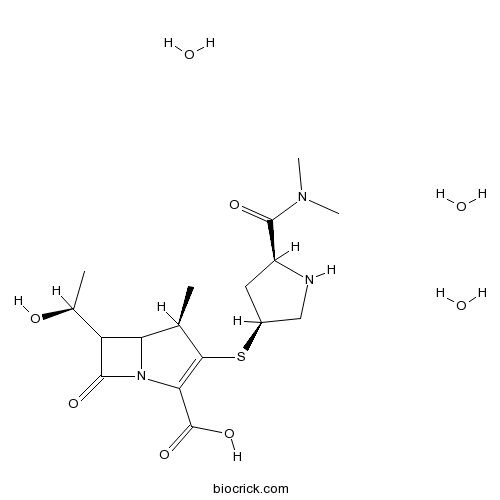
| Chemical Name | (4R)-3-[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl-6-[(1S)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid;trihydrate | ||
| SMILES | CC1C2C(C(=O)N2C(=C1SC3CC(NC3)C(=O)N(C)C)C(=O)O)C(C)O.O.O.O | ||
| Standard InChIKey | CTUAQTBUVLKNDJ-TXBRDXQXSA-N | ||
| Standard InChI | InChI=1S/C17H25N3O5S.3H2O/c1-7-12-11(8(2)21)16(23)20(12)13(17(24)25)14(7)26-9-5-10(18-6-9)15(22)19(3)4;;;/h7-12,18,21H,5-6H2,1-4H3,(H,24,25);3*1H2/t7-,8+,9+,10+,11?,12?;;;/m1.../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Meropenem (trihydrate) is a carbapenem antibiotic, which displaying a broad spectrum of antibacterial activity.
Target: Antibacterial
Meropenem (trihydrate), a new parenteral carbapenem demonstrated increased activity as compared to imipenem against 336 strains of Neisseria gonorrhoeae, 119 strains of Haemophilus influenzae, and 110 strains of H. Ceftriaxone and ciprofloxacin demonstrated activity superior to that of both carbapenems while the activity of ceftazidime was similar to that of meropenem [1]. Meropenem (trihydrate), like imipenem and various experimental penems, may overcome the resistance problems presented by Class I beta-lactamases [2]. Meropenem (trihydrate) was rapidly penetrated to the pleural effusion and was retained for a more prolonged time in the pleural effusion than in the blood of patients with accumulated pleural effusion, and it suggested the usefulness of Meropenem (trihydrate) in antibacterial therapy for patients with pleurisy causing accumulation of pleural effusion [3].
Clinical indications: Appendicitis; Bacterial infection; Bacterial meningitis; Bacterial pneumonia; Bacterial respiratory tract infection; Bacterial skin infection; Bacterial urinary tract infection; Bacteroides fragilis infection; Bacteroides infection; Bacteroides thetaiotaomicron infection; Complicated skin and skin structure infection
FDA Approved Date: July 1996
Toxicity: In mice and rats, large intravenous doses of meropenem (2200-4000 mg/kg) have been associated with ataxia, dyspnea, convulsions, and mortalities. References: | |||||

Meropenem trihydrate Dilution Calculator

Meropenem trihydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2857 mL | 11.4283 mL | 22.8566 mL | 45.7132 mL | 57.1416 mL |
| 5 mM | 0.4571 mL | 2.2857 mL | 4.5713 mL | 9.1426 mL | 11.4283 mL |
| 10 mM | 0.2286 mL | 1.1428 mL | 2.2857 mL | 4.5713 mL | 5.7142 mL |
| 50 mM | 0.0457 mL | 0.2286 mL | 0.4571 mL | 0.9143 mL | 1.1428 mL |
| 100 mM | 0.0229 mL | 0.1143 mL | 0.2286 mL | 0.4571 mL | 0.5714 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Meropenem trihydrate (SM-7338) is an active ingredient carbapenem antibiotic [1].
Meropenem trihydrate has shown the potent effect against gram-negative organisms with the MIC90 values of 0.03μM, 0.06μM, 0.06μM, 0.12μM,0.25μM, 0.12μM, 0.06μM, 0.12μM and 0.25μM for Escherichia coli (30), Klebsiella pneumonia(29), Klebsiella oxytoca (20), Enterobacter aerogenes (14), Enterobacter cloacae (29), Citrobacter freundii (20), Citrobacter diversus (12), proteus mirabilis (15) and Morganella morganii (15), respectively. In addition, Meropenem trihydrate has also been revealed to restrain gram-positive and anaerobic organisms with the MIC90 values of 0.008μM, 4μM and 0.015μM for Streptococcus pyogenes (20), Viridans group streptococci (27), Streptococcus pneumonia (15), respectively. Furthermore, Meropenem trihydrate has shown the different effect of pH on MIC, for example, the mean MIC values of 0.06μM and 0.03μM in pH5.5 and pH7.5, respectively [1].
References:
[1] Neu HC1, Novelli A, Chin NX.In vitro activity and beta-lactamase stability of a new carbapenem, SM-7338.Antimicrob Agents Chemother. 1989 Jul; 33(7):1009-18.
- Fruquintinib(HMPL-013)
Catalog No.:BCC6415
CAS No.:1194506-26-7
- Eliprodil
Catalog No.:BCC7280
CAS No.:119431-25-3
- Loureirin B
Catalog No.:BCN5021
CAS No.:119425-90-0
- Loureirin A
Catalog No.:BCN3671
CAS No.:119425-89-7
- Galanin (1-30) (human)
Catalog No.:BCC6961
CAS No.:119418-04-1
- Licoricesaponin E2
Catalog No.:BCN7894
CAS No.:119417-96-8
- Topotecan hydrochloride
Catalog No.:BCN2604
CAS No.:119413-54-6
- LY2811376
Catalog No.:BCC2102
CAS No.:1194044-20-6
- Przewalskin
Catalog No.:BCN6080
CAS No.:119400-87-2
- Salviolone
Catalog No.:BCN3141
CAS No.:119400-86-1
- 2,5-Dihydroxybenzaldehyde
Catalog No.:BCN6081
CAS No.:1194-98-5
- mAChR-IN-1
Catalog No.:BCC5512
CAS No.:119391-56-9
- Ethyllucidone
Catalog No.:BCN6082
CAS No.:1195233-59-0
- Ceanothic acid acetate
Catalog No.:BCN6083
CAS No.:119533-63-0
- Othonnine
Catalog No.:BCN2061
CAS No.:119565-25-2
- N,N-Dimethylsphingosine
Catalog No.:BCC7959
CAS No.:119567-63-4
- 11-Hydroxygelsenicine
Catalog No.:BCN4761
CAS No.:1195760-68-9
- Dabrafenib (GSK2118436)
Catalog No.:BCC4393
CAS No.:1195765-45-7
- Dabrafenib Mesylate (GSK-2118436)
Catalog No.:BCC1513
CAS No.:1195768-06-9
- 7-Ethyl-10-Hydroxy-Camptothecin
Catalog No.:BCN8386
CAS No.:119577-28-5
- 2-Hydroxyquinoxaline
Catalog No.:BCC8577
CAS No.:1196-57-2
- GSK2190915 sodium salt
Catalog No.:BCC5588
CAS No.:1196070-26-4
- PF-3845
Catalog No.:BCC2326
CAS No.:1196109-52-0
- Olprinone Hydrochloride
Catalog No.:BCC1821
CAS No.:119615-63-3
Spontaneous pyogenic spondylitis caused by Klebsiella pneumoniae.[Pubmed:18552470]
Intern Med. 2008;47(12):1121-4. Epub 2008 Jun 16.
A 72-year-old woman without a history of spinal injury was admitted to our hospital with prolonged severe back pain and high fever. Clinical laboratory findings and magnetic resonance imaging (MRI) revealed severe inflammation of the L2 and L3 lumbar vertebrae. Meropenem trihydrate administration improved her symptoms. Klebsiella pneumoniae isolated from the patient's blood indicated that the organism caused the spontaneous pyogenic spondylitis.
Continuous regional arterial infusion therapy for acute necrotizing pancreatitis due to Mycoplasma pneumoniae infection in a child.[Pubmed:18956223]
Cardiovasc Intervent Radiol. 2009 May;32(3):581-4.
A case of acute necrotizing pancreatitis due to Mycoplasma pneumoniae infection was treated in an 8-year-old girl. She experienced acute pancreatitis during treatment for M. pneumoniae. Contrast-enhanced computed tomographic scan revealed necrotizing pancreatitis. The computed tomographic severity index was 8 points (grade E). A protease inhibitor, ulinastatin, was provided via intravenous infusion but was ineffective. Continuous regional arterial infusion therapy was provided with gabexate mesilate (FOY-007, a protease inhibitor) and Meropenem trihydrate, and the pancreatitis improved. This case suggests that infusion therapy is safe and useful in treating necrotizing pancreatitis in children.
MALDI-TOF MS applied to indirect carbapenemase detection: a validated procedure to clearly distinguish between carbapenemase-positive and carbapenemase-negative bacterial strains.[Pubmed:23584712]
Anal Bioanal Chem. 2013 Jun;405(15):5259-66.
Laboratory identification of carbapenemase-producing clinical isolates is crucial to limit the spread of the bacteria. In this study, we shall first develop the matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) assay in automatic identification of carbapenemase producers. A total of 143 well-characterized isolates were studied. After an incubation of bacteria with Meropenem trihydrate, the mixture was centrifuged and the supernatant analyzed by MALDI-TOF MS. A genetic algorithm model with ClinProTools software was built using spectra of 43 carbapenemase-positive isolates and 40 carbapenemase-negative isolates after 2 h of incubation. This model was externally validated using 60 test isolates. All spectra of supernatants of the carbapenemase-negative isolates showed peak profiles comparable to that of pure meropenem (m/z 384.159, 406.140, and 428.122 of its two sodium salt variants) regardless of the incubation time tested. For the carbapenemase-positive isolates, the specific peak for meropenem at m/z 384.159 disappeared during the incubation time, two products of meropenem degradation were identified with m/z 358.18 (the decarboxylated product) and 380.161 (sodium salt of the decarboxylated product), and other degradation products were observed (native molecule with disrupted amide bond with m/z 402.169, three sodium salt variants with m/z 424.151, 446.133, and 468.115). Sixty test isolates were 100% correctly classified as carbapenemase positive and carbapenemase negative with the genetic algorithm model. MALDI-TOF MS coupled with ClinProTools is capable of rapidly, accurately, and automatically identifying carbapenemase producers.
A validated reverse phase HPLC method for the determination of disodium EDTA in meropenem drug substance with UV-detection using precolumn derivatization technique.[Pubmed:21760705]
Anal Chem Insights. 2011;6:7-14.
This paper deals with development and validation of a high performance liquid chromatographic method for the quantitative determination of disodium EDTA (Ethylenediaminetetraacetic acid) in Meropenem active pharmaceutical ingredient (API). EDTA was derivatized with Ferric chloride solution by heating at 70 degrees C in water bath for about 20 minutes and the chromatographic separation achieved by injecting 100 muL of the derivatized mixture into a Waters HPLC system with photodiode array detector using a Phenomenex Luna C18(2) column (250 x 4.6 mm), 5 mu. The mobile phase consisting of 5% methanol and 95% of 0.7 g/L solution of Tetra butyl ammonium bromide and 4.6 g/L solution of sodium acetate trihydrate in water (pH adjusted to 4.0 with the help of acetic acid glacial) and a flow rate of 1 milliliter/minute. EDTA eluted at approximately 6 minutes. The method was suitably validated with respect to specificity, linearity of response, precision, accuracy, ruggedness, stability in analytical solution, limit of quantitation and detection and robustness for its intended use.
Impact of antibacterial drugs on human serum paraoxonase-1 (hPON1) activity: an in vitro study.[Pubmed:25183328]
Asian Pac J Trop Biomed. 2014 Aug;4(8):603-9.
OBJECTIVE: To investigate the in vitro effects of the antibacterial drugs, Meropenem trihydrate, piperacillin sodium, and cefoperazone sodium, on the activity of human serum paraoxonase (hPON1). METHODS: hPON1 was purified from human serum using simple chromatographic methods, including DEAE-Sephadex anion exchange and Sephadex G-200 gel filtration chromatography. RESULTS: The three antibacterial drugs decreased in vitro hPON1 activity. Inhibition mechanisms Meropenem trihydrate was noncompetitive while piperacillin sodium and cefoperazone sodium were competitive. CONCLUSIONS: Our results showed that antibacterial drugs significantly inhibit hPON1 activity, both in vitro, with rank order Meropenem trihydrate piperacillin sodium cefoperazone sodium in vitro.


