JMV 449Potent neurotensin receptor agonist CAS# 139026-66-7 |
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- GDC-0449 (Vismodegib)
Catalog No.:BCC1285
CAS No.:879085-55-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 139026-66-7 | SDF | Download SDF |
| PubChem ID | 164415 | Appearance | Powder |
| Formula | C38H66N8O7 | M.Wt | 746.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 0.80 mg/ml in water | ||
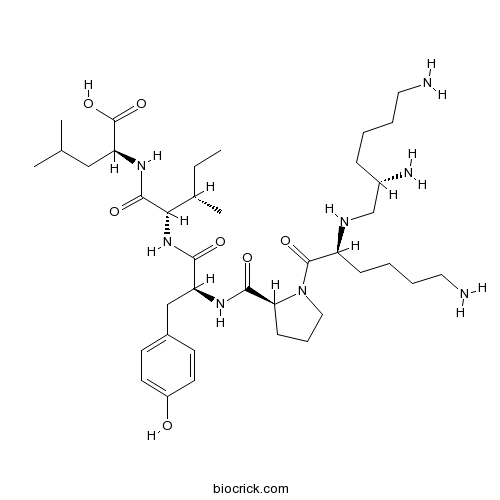
| Sequence | KKPYIL (Modifications: Lys-1 - Lys-2 peptide bond replace with Ψ(CH2-NH)) | ||
| Chemical Name | (2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-1-[(2S)-6-amino-2-[[(2S)-2,6-diaminohexyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-methylpentanoyl]amino]-4-methylpentanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(CC(C)C)C(=O)O)NC(=O)C(CC1=CC=C(C=C1)O)NC(=O)C2CCCN2C(=O)C(CCCCN)NCC(CCCCN)N | ||
| Standard InChIKey | TZCYVPLNMOJUIL-GULBXNHPSA-N | ||
| Standard InChI | InChI=1S/C38H66N8O7/c1-5-25(4)33(36(50)44-31(38(52)53)21-24(2)3)45-34(48)30(22-26-14-16-28(47)17-15-26)43-35(49)32-13-10-20-46(32)37(51)29(12-7-9-19-40)42-23-27(41)11-6-8-18-39/h14-17,24-25,27,29-33,42,47H,5-13,18-23,39-41H2,1-4H3,(H,43,49)(H,44,50)(H,45,48)(H,52,53)/t25-,27-,29-,30-,31-,32-,33-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, metabolically stable neurotensin receptor agonist peptide (IC50 = 0.15 nM for inhibition of [125I]-NT binding to neonatal mouse brain; EC50 = 1.9 nM for contraction of guinea pig ileum). Produces long-lasting hypothermic, neuroprotective and analgesic effects in mice following central administration in vivo. |

JMV 449 Dilution Calculator

JMV 449 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oplodiol
Catalog No.:BCN6204
CAS No.:13902-62-0
- Catalpin
Catalog No.:BCN6205
CAS No.:1390-72-3
- Carmine
Catalog No.:BCN2223
CAS No.:1390-65-4
- 3,4-Dihydroxybenzaldehyde
Catalog No.:BCN6214
CAS No.:139-85-5
- Ziprasidone hydrochloride monohydrate
Catalog No.:BCC2072
CAS No.:138982-67-9
- Capsazepine
Catalog No.:BCC1451
CAS No.:138977-28-3
- Coronarin D ethyl ether
Catalog No.:BCN6203
CAS No.:138965-89-6
- Isocoronarin D
Catalog No.:BCN6202
CAS No.:138965-88-5
- Rutaretin
Catalog No.:BCN4710
CAS No.:13895-92-6
- Ibandronate sodium
Catalog No.:BCC4665
CAS No.:138926-19-9
- Entadamide-A-β-D-glucopyranoside
Catalog No.:BCN8452
CAS No.:138916-58-2
- Brinzolamide
Catalog No.:BCC2313
CAS No.:138890-62-7
- 2-PMDQ
Catalog No.:BCC6726
CAS No.:139047-55-5
- Anemarsaponin B
Catalog No.:BCN6289
CAS No.:139051-27-7
- L-689,560
Catalog No.:BCC6774
CAS No.:139051-78-8
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- (-)-Heraclenol
Catalog No.:BCN7682
CAS No.:139079-42-8
- Tristin
Catalog No.:BCN4709
CAS No.:139101-67-0
- PF 4800567 hydrochloride
Catalog No.:BCC7904
CAS No.:1391052-28-0
- Vigabatrin Hydrochloride
Catalog No.:BCC5198
CAS No.:1391054-02-6
- Zanamivir
Catalog No.:BCC4946
CAS No.:139110-80-8
- BM-1074
Catalog No.:BCC2235
CAS No.:1391108-10-3
- Isomurralonginol acetate
Catalog No.:BCN4708
CAS No.:139115-59-6
- Tripterifordin
Catalog No.:BCN6206
CAS No.:139122-81-9
JMV 449: a pseudopeptide analogue of neurotensin-(8-13) with highly potent and long-lasting hypothermic and analgesic effects in the mouse.[Pubmed:1425958]
Eur J Pharmacol. 1992 Aug 25;219(2):327-9.
We recently reported that H-Lys psi (CH2NH)Lys-Pro-Tyr-Ile-Leu-OH (JMV 449), a pseudopeptide analogue of neurotensin-(8-13) with a reduced CH2NH bond in position 8-9, was about 3 times more potent than neurotensin in binding to mouse brain membranes and in contracting the guinea-pig ileum, and was markedly more resistant to degradation than neurotensin when exposed to rat brain membranes. In the present study, we compared the time courses and dose-response relationships for the ability of i.c.v. injected neurotensin and JMV 449 to elicit hypothermia and analgesia (tail-flick test) in the mouse. The results show that the pseudopeptide analogue behaved as a highly potent and long-lasting neurotensin agonist in these two in vivo bioassays. The analogue should prove very useful for studying the effects of chronic neurotensin receptor stimulation in vitro and in vivo.
Neuroprotective effect of the neurotensin analogue JMV-449 in a mouse model of permanent middle cerebral ischaemia.[Pubmed:14623134]
Neurosci Lett. 2003 Nov 20;351(3):173-6.
The neuroprotective effect of the neurotensin analogue H-Lys-psi(CH2NH)Lys-Pro-Tyr-Ile-Leu-OH (JMV-449) was assessed in a mouse model of permanent distal middle cerebral artery occlusion. Mice were injected with 0.6 nmol JMV-449 or vehicle i.c.v. immediately after ischaemia. The core temperature declined by 6-7 degrees C after 30 min and the hypothermia persisted for 4-5 h. JMV-449 treatment was able to reduce the infarct volume significantly both at 24 h and 14 days after onset of ischaemia. No neuroprotective effect could be seen if the mice were kept normothermic after the JMV-449 treatment suggesting that the neuroprotective effect is mediated via the hypothermia.
Reduced peptide bond pseudopeptide analogues of neurotensin: binding and biological activities, and in vitro metabolic stability.[Pubmed:1812009]
Eur J Pharmacol. 1991 Nov 26;205(2):191-8.
A series of pseudopeptide analogues of neurotensin was produced by systematically replacing the five peptide bonds in neurotensin-(8-13) with CH2NH (psi, reduced) bonds. All these analogues were synthesized with a free amino terminus (H derivatives) and with a N-terminal tert-butyloxycarbonyl group (Boc derivatives). The compounds were screened in vitro for agonist or antagonist activity and for metabolic stability by testing (1) their ability to inhibit the binding of radiolabelled neurotensin to homogenates of newborn mouse brain; (2) their ability to contract isolated guinea-pig ileum preparations; and (3) their degradation in the presence of rat brain homogenates. All the analogues bound to the mouse brain neurotensin receptor and all exhibited agonist activity in the guinea-pig ileum assay. Only the H- and Boc-[psi 8,9] derivatives were at least as potent as their parent compounds neurotensin-(8-13) and Boc-neurotensin-(8-13) in the binding and biological assays. All the other pseudopeptide analogues with reduced bonds at position 9-10, 10-11, 11-12 and 12-13 showed a marked reduction in potency ranging from 2 to 4 orders of magnitude. All the derivatives that were protected at their N terminus either by the presence of a Boc group or by the presence of a reduced bond at position 8-9 and 9-10 were slowly degraded by rat brain homogenates. The other derivatives were, in contrast, quite rapidly degraded. There was a good correlation between binding and biological potencies for those analogues that were resistant to degradation. Interestingly, the degradation-resistant H-[psi 8,9] compound exhibited higher binding and biological potency then neurotensin. It is therefore expected that this analogue will produce highly potent and long-lasting neurotensin-like effects in vivo, and preliminary experiments indicate that this is indeed the case.


