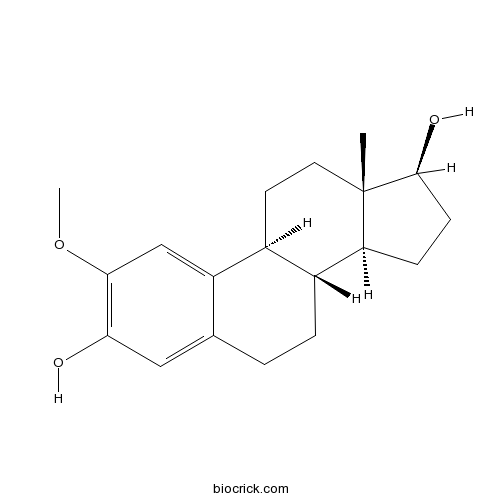
2-Methoxyestradiol (2-MeOE2)Apoptotic, antiproliferative and antiangiogenic agent CAS# 362-07-2 |
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure
3D structure
| Cas No. | 362-07-2 | SDF | Download SDF |
| PubChem ID | 66414 | Appearance | Powder |
| Formula | C19H26O3 | M.Wt | 302.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 2-ME2; NSC-659853 | ||
| Solubility | DMSO : ≥ 100 mg/mL (330.68 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (8R,9S,13S,14S,17S)-2-methoxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol | ||
| SMILES | CC12CCC3C(C1CCC2O)CCC4=CC(=C(C=C34)OC)O | ||
| Standard InChIKey | CQOQDQWUFQDJMK-SSTWWWIQSA-N | ||
| Standard InChI | InChI=1S/C19H26O3/c1-19-8-7-12-13(15(19)5-6-18(19)21)4-3-11-9-16(20)17(22-2)10-14(11)12/h9-10,12-13,15,18,20-21H,3-8H2,1-2H3/t12-,13+,15-,18-,19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Apoptotic, antiproliferative and antiangiogenic agent, in vitro and in vivo; acts via an estrogen receptor-independent mechanism. Induces p53-induced apoptosis via two pathways: activation of p38 and NF-κB; and activation of JNK and AP-1 leading to Bcl-2 phosphorylation. Also upregulates death receptor 5 and binds to tubulin, inhibiting its assembly. |

2-Methoxyestradiol (2-MeOE2) Dilution Calculator

2-Methoxyestradiol (2-MeOE2) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3068 mL | 16.5338 mL | 33.0677 mL | 66.1354 mL | 82.6692 mL |
| 5 mM | 0.6614 mL | 3.3068 mL | 6.6135 mL | 13.2271 mL | 16.5338 mL |
| 10 mM | 0.3307 mL | 1.6534 mL | 3.3068 mL | 6.6135 mL | 8.2669 mL |
| 50 mM | 0.0661 mL | 0.3307 mL | 0.6614 mL | 1.3227 mL | 1.6534 mL |
| 100 mM | 0.0331 mL | 0.1653 mL | 0.3307 mL | 0.6614 mL | 0.8267 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
2-Methoxyestradiol (2-MeOE2), an endogenous metabolite of 17β-estradiol (E2), is an inhibitor of microtubule assembly that inhibits the polymerization of tubulin and interferes with mitotic spindle dynamics leading to the blockage of mitosis of human cancer cells which lack estrogen receptors in metaphase. 2-MeOE2 is also an inhibitor of tumor growth and angiogenesis. Study results have shown that 2-MeOE2 induces mammalian cell transformation and genotoxicity in Syrian hamster embryo (SHE) fibroblasts through concentration-dependent inhibition of cell growth. Moreover, 2-MeOE2 has demonstrated anti-proliferative activity against estrogen-responsive breast cancer cell line MCF-7 and subsequent inhibition of the growth of tumors subcutaneously inoculated in mice.
Reference
Takeki Tsutsui, Yukiko Tamura, Makoto Hagiwara, Takashi Miyachi, Hirohito Hikiba, Chikahiro Kubo and J. Carl Barret. Induction of mammalian cell transformation and genotoxicity by 2-methoxyestradiol, an endogenous metabolite of estrogen. Carcinogensis 2000; 21(4): 735-740
Hesham Attalla, Tomi P. Makela, Herman Adlercreutz and Leif C. Anderson. 2-Methoxyestradiol arrests cells in mitosis without depolymerizing tubulin. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS 1996; 228: 467-473
- Genistein 7,4'-di-O-beta-D-glucopyranoside
Catalog No.:BCN7835
CAS No.:36190-98-4
- 3'-O-Methylorobol
Catalog No.:BCN5318
CAS No.:36190-95-1
- 3-Acetyl-2,5-dichlorothiophene
Catalog No.:BCC8602
CAS No.:36157-40-1
- Blumenol B
Catalog No.:BCN5317
CAS No.:36151-01-6
- Kobusin
Catalog No.:BCN7563
CAS No.:36150-23-9
- Dehydroleucodine
Catalog No.:BCN6897
CAS No.:36150-07-9
- Saxalin
Catalog No.:BCC8357
CAS No.:36150-06-8
- Mullilam diol
Catalog No.:BCN5316
CAS No.:36150-04-6
- Phytin
Catalog No.:BCN1285
CAS No.:3615-82-5
- alpha-L-Rhamnose
Catalog No.:BCN2592
CAS No.:3615-41-6
- D-(+)-Fucose
Catalog No.:BCN6432
CAS No.:3615-37-0
- JDTic
Catalog No.:BCC1670
CAS No.:361444-66-8
- NVP 231
Catalog No.:BCC4244
CAS No.:362003-83-6
- Columbamine
Catalog No.:BCN2722
CAS No.:3621-36-1
- Jatrorrhizine
Catalog No.:BCN5319
CAS No.:3621-38-3
- Cyclo(D-Leu-L-Pro)
Catalog No.:BCN4028
CAS No.:36238-67-2
- TC 1
Catalog No.:BCC7450
CAS No.:362512-81-0
- (±)-SLV 319
Catalog No.:BCC7954
CAS No.:362519-49-1
- Clemaphenol A
Catalog No.:BCN7834
CAS No.:362606-60-8
- Pitolisant
Catalog No.:BCC1862
CAS No.:362665-56-3
- Pitolisant oxalate
Catalog No.:BCC1864
CAS No.:362665-57-4
- Hederasaponin B
Catalog No.:BCN1085
CAS No.:36284-77-2
- Prostaglandin E2
Catalog No.:BCC7316
CAS No.:363-24-6
- Broussonol E
Catalog No.:BCN7996
CAS No.:363134-28-5
Endogenous estrogen metabolites as biomarkers for endometrial cancer via a novel method of liquid chromatography-mass spectrometry with hollow fiber liquid-phase microextraction.[Pubmed:24722971]
Horm Metab Res. 2015 Feb;47(2):158-64.
Increased levels of endogenous estrogens and their metabolites are well-known risk factors of endometrial cancer. The aim of this study was to quantitatively assess the potential for estrogen metabolites to serve as biomarkers of endometrial carcinogenesis. The following estrogen metabolites were evaluated: 2-hydroxyestradiol (2-OHE2), 2-hydroxyestrone (2-OHE1), 4-hydroxyestradiol (4-OHE2), 4-hydroxyestrone (4-OHE1), 16alpha-hydroxyestrone (16alpha-OHE1), 2-Methoxyestradiol (2-MeOE2), and 2-methoxyestrone (2-MeOE1). The low content of estrogen metabolites in urine makes their measurement difficult. To address this issue, we developed a rapid, sensitive, specific, and accurate liquid chromatography-mass spectrometry (LC-MS) method, with hollow fiber liquid-phase micro-extraction (HF-LPME) for an enriched pretreatment of the sample and for the simultaneous quantification of estrogens and their metabolites in the urine samples of 23 post-menopausal female endometrial cancer patients and 23 post-menopausal healthy female controls. The levels of estrogens were found to differ between the endometrial cancer patients and the controls. The level of 4-OHE2 was elevated in patients compared with the controls, while the levels of 2-MeOE1 and 2-MeOE2 were reduced in the endometrial cancer group. The results of this study indicate an imbalance of estrogen metabolites in endometrial carcinogenesis, and that the elevation of 4-OHE2 may be used as a potential biomarker for the risk assessment of estrogen-induced endometrial cancer.
HIF-1alpha signaling activation by post-ischemia treatment with astragaloside IV attenuates myocardial ischemia-reperfusion injury.[Pubmed:25238237]
PLoS One. 2014 Sep 19;9(9):e107832.
In this study, we evaluated the effect of astragaloside IV (Ast IV) post-ischemia treatment on myocardial ischemia-reperfusion (IR) injury (IRI). We also examined whether hypoxia inducible factor-1alpha (HIF-1alpha) and its downstream gene-inducible nitric oxide (NO) synthase (iNOS) play roles in the cardioprotective effect of Ast IV. Cultured cardiomyocytes and perfused isolated rat hearts were exposed to Ast IV during reperfusion in the presence or absence of the HIF-1alpha inhibitor 2-Methoxyestradiol (2-MeOE2). The post-ischemia treatment with Ast IV protected cardiomyocytes from the apoptosis and death induced by simulated IRI (SIRI). Additionally, in cardiomyocytes, 2-MeOE2 and HIF-1alpha siRNA treatment each not only abolished the anti-apoptotic effect of post-ischemia treatment with Ast IV but also reversed the upregulation of HIF-1alpha and iNOS expression. Furthermore, after treatment with Ast IV, post-ischemic cardiac functional recovery and lactate dehydrogenase (LDH) release in the coronary flow (CF) were improved, and the myocardial infarct size was decreased. Moreover, the number of apoptotic cells was reduced, and the upregulation of the anti-apoptotic protein Bcl2 and downregulation of the pro-apoptotic protein Caspase3 were reversed. 2-MeOE2 reversed these effects of Ast IV on IR-injured hearts. These results suggest that post-ischemia treatment with Ast IV can attenuate IRI by upregulating HIF-1alpha expression, which transmits a survival signal to the myocardium.
Methylene blue-induced neuronal protective mechanism against hypoxia-reoxygenation stress.[Pubmed:26047733]
Neuroscience. 2015 Aug 20;301:193-203.
UNLABELLED: Brain ischemia and reperfusion (I/R) injury occurs in various pathological conditions, but there is no effective treatment currently available in clinical practice. Methylene blue (MB) is a century-old drug with a newly discovered protective function in the ischemic stroke model. In the current investigation we studied the MB-induced neuroprotective mechanism focusing on stabilization and activation of hypoxia-inducible factor-1alpha (HIF-1alpha) in an in vitro oxygen and glucose deprivation (OGD)-reoxygenation model. METHODS: HT22 cells were exposed to OGD (0.1% O2, 6h) and reoxygenation (21% O2, 24h). Cell viability was determined with the calcein AM assay. The dynamic change of intracellular O2 concentration was monitored by fluorescence lifetime imaging microscopy (FLTIM). Glucose uptake was quantified using the 2-[N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)Amino]-2-Deoxy-d-Glucose (2-NBDG) assay. ATP concentration and glycolytic enzyme activity were examined by spectrophotometry. Protein content changes were measured by immunoblot: HIF-1alpha, prolyl hydroxylase 2 (PHD2), erythropoietin (EPO), Akt, mTOR, and PIP5K. The contribution of HIF-1alpha activation in the MB-induced neuroprotective mechanism was confirmed by blocking HIF-1alpha activation with 2-methoxyestradiol-2 (2-MeOE2) and by transiently transfecting constitutively active HIF-1alpha. RESULTS: MB increases cell viability by about 50% vs. OGD control. Compared to the corresponding control, MB increases intracellular O2 concentration and glucose uptake as well as the activities of hexokinase and G-6-PDH, and ATP concentration. MB activates the EPO signaling pathway with a corresponding increase in HIF-1alpha. Phosphorylation of Akt was significantly increased with MB treatment followed by activation of the mTOR pathway. Importantly, we observed, MB increased nuclear translocation of HIF-1alpha vs. control (about three folds), which was shown by a ratio of nuclear:cytoplasmic HIF-1alpha protein content. CONCLUSION: We conclude that MB protects the hippocampus-derived neuronal cells against OGD-reoxygenation injury by enhancing energy metabolism and increasing HIF-1alpha protein content accompanied by an activation of the EPO signaling pathway.
2-Methoxyestradiol enhances radiosensitivity in radioresistant melanoma MDA-MB-435R cells by regulating glycolysis via HIF-1alpha/PDK1 axis.[Pubmed:28339028]
Int J Oncol. 2017 May;50(5):1531-1540.
HIF-1alpha overexpression is associated with radio-resistance of various cancers. A radioresistant human melanoma cell model MDA-MB-435R (435R) was established by us previously. Compared with the parental cells MDA-MB435 (435S), an elevated level of HIF-1alpha expression in 435R cells was demonstrated in our recent experiments. Therefore, in the current study, we sought to determine whether selective HIF-1alpha inhibitors could radiosensitize the 435R cells to X-ray, and to identify the potential mechanisms. Our data demonstrated that inhibition of HIF-1alpha with 2-Methoxyestradiol (2-MeOE2) significantly enhanced radiosensitivity of 435R cells. 2-MeOE2 increased DNA damage and ratio of apoptosis cells induced by irradiation. Whereas, cell proliferation and the expression of pyruvate dehydrogenase kinase 1 (PDK1) were decreased after 2-MeOE2 treatment. The change of expression of GLUT1, LDHA and the cellular ATP level and extracellular lactate production indicates that 2-MeOE2 suppressed glycolytic state of 435R cells. In addition, the radioresistance, glycolytic state and cell proliferation of 435R cells were also decreased after inhibiting pyruvate dehydrogenase kinase 1 (PDK1) with dichloroacetate (DCA). DCA could also increase DNA damage and ratio of apoptotic cells induced by irradiation. These results also suggest that inhibition of HIF-1alpha with 2-MeOE2 sensitizes radioresistant melanoma cells 435R to X-ray irradiation through targeting the glycolysis that is regulated by PDK1. Selective inhibitors of HIF-1alpha and glycolysis are potential drugs to enhance radio-sensitivity of melanoma cells.
Folic acid attenuates cobalt chloride-induced PGE2 production in HUVECs via the NO/HIF-1alpha/COX-2 pathway.[Pubmed:28624453]
Biochem Biophys Res Commun. 2017 Aug 19;490(2):567-573.
Prostaglandin E2 (PGE2), an important lipid inflammatory mediator involved in the progression of vascular diseases, can be induced by hypoxia in many cell types. While folic acid has been shown to protect against inflammation in THP-1 cells during hypoxia and hypoxia-induced endothelial cell injury, whether it might do so by attenuating PGE2 production remains unclear. To investigate this we constructed a hypoxia-induced injury model by treating human umbilical vein endothelial cells (HUVECs) with cobalt chloride (CoCl2), which mimics the effects of hypoxia. In CoCl2-treated HUVECs, folic acid significantly attenuated PGE2 production and increased vasoprotective nitric oxide (NO) content. Folic acid also decreased cyclooxygenase-2 (COX-2) and hypoxia-inducible factor 1-alpha (HIF-1alpha) expression and altered endothelial nitric oxide synthase (eNOS) signaling by increasing p-eNOS((Ser1177)) and decreasing p-eNOS((Thr495)) in a dose-dependent manner. Further investigation of the pathway demonstrated that treatment with 2-Methoxyestradiol (2-MeOE2) and celecoxib both decreased CoCl2-induced COX-2 expression but only 2-MeOE2 decreased HIF-1alpha expression. The ability of folic acid to down-regulate HIF-1alpha and COX-2 protein levels was dramatically abrogated by L-NAME treatment, which also decreased eNOS mRNA and NO production. The NO donor sodium nitroprusside also dose-dependently down-regulated HIF-1alpha and COX-2 protein levels. Overall, these findings suggest a novel application for folic acid in attenuating CoCl2-induced PGE2 production in HUVECs via regulation of the NO/HIF-1alpha/COX-2 pathway.
2-methoxyestradiol up-regulates death receptor 5 and induces apoptosis through activation of the extrinsic pathway.[Pubmed:12543804]
Cancer Res. 2003 Jan 15;63(2):468-75.
2-Methoxyestradiol (2ME2), a natural metabolite of estradiol, is a potent antitumor and antiangiogenic agent. In vitro, 2ME2 inhibits the proliferation of a wide variety of cell lines and primary cultures, and in numerous models in vivo, it has been shown to be an effective inhibitor of tumor growth and angiogenesis. 2ME2 is currently in several Phase I and Phase II clinical trials under the name Panzem. Although various molecular targets have been proposed for this compound, the mechanism by which 2ME2 exerts its effects is still uncertain. This study shows that 2ME2 uses the extrinsic pathway for induction of apoptosis. 2ME2 treatment results in up-regulation of death receptor 5 (DR5) protein expression in vitro and in vivo and renders cells more sensitive to the cytotoxic activities of the DR5 ligand tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). 2ME2-induced apoptosis requires caspase activation and kinetic studies show the sequential activation of caspase-8, caspase-9, and caspase-3. Blockage of death receptor signaling by expression of dominant-negative Fas-associated death domain severely attenuates the ability of 2ME2 to induce apoptosis. Because 2ME2 administration has not manifested dose-limiting toxicity in the clinic, DR5 expression may serve as a surrogate marker for biological response.
Roles of p38- and c-jun NH2-terminal kinase-mediated pathways in 2-methoxyestradiol-induced p53 induction and apoptosis.[Pubmed:12807754]
Carcinogenesis. 2003 Jun;24(6):1067-75.
As 2-methoxyestradiol (2-ME), an endogenous estrogen metabolite, has been established to cause apoptosis of prostate cancer cells, the downstream effectors of the signaling remain unclear. In the current study, we investigated molecular mechanisms by which 2-ME induces apoptosis in human prostate cancer cell line, LNCaP. It was found that 2-ME mediates apoptosis through p53 induction. Nuclear factor kappaB (NFkappaB) was activated by 2-ME and closely regulated by the mitogen-activated protein kinase, p38. Inhibition of p38 or NFkappaB resulted in suppression of p53 induction and apoptosis. Moreover, we demonstrated that 2-ME activates the c-jun NH2-terminal kinase (JNK)/activation protein (AP)-1 pathway. Interestingly, inhibition of JNK strongly reduced Bcl-2 phosphorylation by 2-ME as well as p53 induction, and almost completely suppressed 2-ME-induced apoptosis. Androgen stimulation with dihydrotestosterone, a major endogenous metabolite of testosterone, also significantly inhibited p38/NFkappaB and JNK/AP-1 activation and apoptosis. The results suggest that not only p53 induction through p38/JNK-dependent NFkappaB/AP-1 activation but also JNK-dependent Bcl-2 phosphorylation are required for 2-ME-induced apoptosis; moreover, inhibition of these pathways may be involved in androgen-mediated resistance to apoptosis.
2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta.[Pubmed:12097276]
Cancer Res. 2002 Jul 1;62(13):3691-7.
2-Methoxyestradiol (2ME(2)) is an endogenous metabolite of 17beta-estradiol (E(2)) that arises from the hydroxylation and subsequent methylation at the 2-position. In vitro 2ME(2) inhibits a large variety of tumor and nontumor cell lines from diverse origins, as well as several stages of the angiogenic cascade. In vivo it has been shown to be a very effective inhibitor of tumor growth and angiogenesis in numerous models. Although various molecular targets have been proposed for this compound, the mechanism of action is still uncertain. As this molecule emerges as a drug candidate it is important to assess the estrogen receptors (ERs) as molecular targets for 2ME(2). The purpose of this study was to investigate whether 2ME(2) is able to engage ERs as an agonist and whether its antiproliferative activities are mediated through ERs. We confirm that 2ME(2) has a lower binding affinity for ERalpha as compared with E(2) and other E(2) metabolites and antagonists, and we demonstrate that the affinity of 2ME(2) for ERbeta is even lower. When assessed in the presence of galangin, a cytochrome P450 enzyme inhibitor, at concentrations at which 2ME(2) interacts with ERalpha in an in vitro binding assay, it does not stimulate the proliferation of an estrogen-dependent breast carcinoma cell line. Similar IC(50) values for inhibition of proliferation and induction of apoptosis are obtained in estrogen-dependent and estrogen-independent human breast cancer cell lines, irrespective of the expression of ERalpha and ERbeta. Moreover, the estrogen antagonist ICI 182,780 does not inhibit the antiproliferative activity of 2ME(2). In E(2)-responsive cells such as MCF-7 and human umbilical vascular endothelial cells, high levels of E(2) inhibit the antiproliferative activity of ICI 182,780 but not of 2ME(2). Collectively, these results suggest that 2ME(2) is distinct among estradiol metabolites because of its inability to engage ERs as an agonist, and its unique antiproliferative and apoptotic activities are mediated independently of ERalpha and ERbeta.
2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site.[Pubmed:8171020]
Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3964-8.
A metabolite of estradiol, 2-methoxyestradiol (2ME), inhibits angiogenesis in the chicken embryo chorioallantoic membrane assay. Since 2ME causes mitotic perturbations, we examined its interactions with tubulin. In our standard 1.0 M glutamate system (plus 1.0 mM MgCl2 at 37 degrees C), superstoichiometric concentrations (relative to tubulin) of 2ME inhibited the nucleation and propagation phases of tubulin assembly but did not affect the reaction extent. Although polymer formed in the presence of 2ME was more cold-stable than control polymer, morphology was little changed. Under suboptimal reaction conditions (0.8 M glutamate/no MgCl2 at 26 degrees C), substoichiometric 2ME totally inhibited polymerization. No other estrogenic compound was as effective as 2ME as an inhibitor of polymerization or of the binding of colchicine to tubulin. Inhibition of colchicine binding was competitive (Ki, 22 microM). Thus, a mammalian metabolite of estradiol binds to the colchicine site of tubulin and, depending on reaction conditions, either inhibits assembly or seems to be incorporated into a polymer with altered stability properties.


