IsodiospyrinCAS# 20175-84-2 |
Quality Control & MSDS
Number of papers citing our products

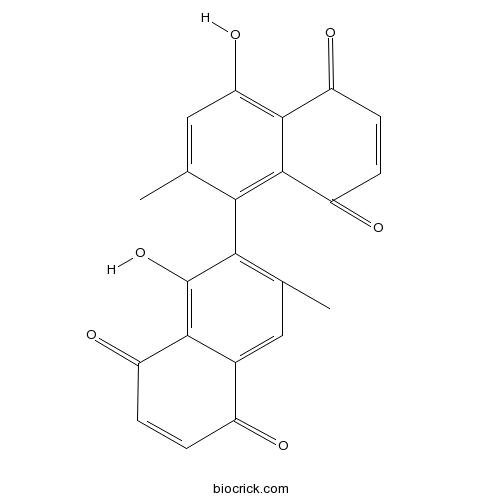
Chemical structure

3D structure
| Cas No. | 20175-84-2 | SDF | Download SDF |
| PubChem ID | 99298 | Appearance | Red powder |
| Formula | C22H14O6 | M.Wt | 374.4 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-6-(4-hydroxy-2-methyl-5,8-dioxonaphthalen-1-yl)-7-methylnaphthalene-1,4-dione | ||
| SMILES | CC1=CC(=C2C(=O)C=CC(=O)C2=C1C3=C(C=C4C(=O)C=CC(=O)C4=C3O)C)O | ||
| Standard InChIKey | OEEOHKZVBKYMBA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H14O6/c1-9-7-11-12(23)3-4-13(24)19(11)22(28)18(9)17-10(2)8-16(27)20-14(25)5-6-15(26)21(17)20/h3-8,27-28H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isodiospyrin is a novel human DNA topoisomerase I inhibitor, it exhibits cytotoxic activity to tumor cell lines. 2. Isodiospyrin has antibacterial activity, the minimum inhibitory concentrations (MICs) against Gram-positive bacteria ranged from 0.78 to 50 microg/mL. 3. Isodiospyrin shows high antifungal activity against P. obscurans at 30uM with 81.4% growth inhibition, and moderate activity against P. viticola (36.6%). |
| Targets | Topoisomerase | Antifection |

Isodiospyrin Dilution Calculator

Isodiospyrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6709 mL | 13.3547 mL | 26.7094 mL | 53.4188 mL | 66.7735 mL |
| 5 mM | 0.5342 mL | 2.6709 mL | 5.3419 mL | 10.6838 mL | 13.3547 mL |
| 10 mM | 0.2671 mL | 1.3355 mL | 2.6709 mL | 5.3419 mL | 6.6774 mL |
| 50 mM | 0.0534 mL | 0.2671 mL | 0.5342 mL | 1.0684 mL | 1.3355 mL |
| 100 mM | 0.0267 mL | 0.1335 mL | 0.2671 mL | 0.5342 mL | 0.6677 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (S)-3,4-DCPG
Catalog No.:BCC7012
CAS No.:201730-11-2
- (R)-3,4-DCPG
Catalog No.:BCC7046
CAS No.:201730-10-1
- Triptocalline A
Catalog No.:BCN6783
CAS No.:201534-10-3
- Triptocallic acid D
Catalog No.:BCN4882
CAS No.:201534-09-0
- Fmoc-D-Pen(Trt)-OH
Catalog No.:BCC3309
CAS No.:201532-01-6
- Fmoc-Pen(Trt)-OH
Catalog No.:BCC3306
CAS No.:201531-88-6
- Deferasirox
Catalog No.:BCC3924
CAS No.:201530-41-8
- Dilazep dihydrochloride
Catalog No.:BCC6660
CAS No.:20153-98-4
- Fmoc-Asn-ol
Catalog No.:BCC2586
CAS No.:201484-12-0
- Boc-Dap(Boc)-OH.DCHA
Catalog No.:BCC2664
CAS No.:201472-68-6
- PKI 14-22 amide, myristoylated
Catalog No.:BCC8087
CAS No.:201422-03-9
- Talarozole
Catalog No.:BCC1980
CAS No.:201410-53-9
- Ac-Phe-OH
Catalog No.:BCC3005
CAS No.:2018-61-3
- Z-Leu-OH
Catalog No.:BCC2766
CAS No.:2018-66-8
- Tenuifolin
Catalog No.:BCN5005
CAS No.:20183-47-5
- Pisatin
Catalog No.:BCN3912
CAS No.:20186-22-5
- Magnolioside
Catalog No.:BCN2832
CAS No.:20186-29-2
- Ombuoside
Catalog No.:BCN3711
CAS No.:20188-85-6
- PTAC oxalate
Catalog No.:BCC6217
CAS No.:201939-40-4
- Epicatechin pentaacetate
Catalog No.:BCN4884
CAS No.:20194-41-6
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- Kaempferol 7-O-rhamnoside
Catalog No.:BCN6489
CAS No.:20196-89-8
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Bilastine
Catalog No.:BCC5263
CAS No.:202189-78-4
Antifungal metabolites from the roots of Diospyros virginiana by overpressure layer chromatography.[Pubmed:22162171]
Chem Biodivers. 2011 Dec;8(12):2331-40.
A preparative overpressure layer chromatography (OPLC) method was successfully used for the separation of two new natural compounds, 4-hydroxy-5,6-dimethoxynaphthalene-2-carbaldehyde (1) and 12,13-didehydro-20,29-dihydrobetulin (2) together with nine known compounds, including 7-methyljuglone (3), diospyrin (4), Isodiospyrin (5), shinanolone (6), lupeol (7), betulin (8), betulinic acid (9), betulinaldehyde (10), and ursolic acid (11) from the acetone extract of the roots of Diospyros virginiana. Their identification was accomplished by 1D- and 2D-NMR spectroscopy and HR-ESI-MS methods. All the isolated compounds were evaluated for their antifungal activities against Colletotrichum fragariae, C. gloeosporioides, C. acutatum, Botrytis cinerea, Fusarium oxysporum, Phomopsis obscurans, and P. viticola using in vitro micro-dilution broth assay. The results indicated that compounds 3 and 5 showed high antifungal activity against P. obscurans at 30 muM with 97.0 and 81.4% growth inhibition, and moderate activity against P. viticola (54.3 and 36.6%). It appears that an optimized OPLC system offers a rapid and efficient method of exploiting bioactive natural products.
Antibacterial activity of diospyrin, isodiospyrin and bisisodiospyrin from the root of Diospyros piscatoria (Gurke) (Ebenaceae).[Pubmed:10685108]
Phytother Res. 2000 Mar;14(2):112-7.
Two dimeric naphthoquinones, diospyrin and Isodiospyrin, isolated from the root of Diospyros piscatoria (Gurke), a common ingredient in several folk medicines, have been shown to have a broad spectrum of antibacterial activity. The minimum inhibitory concentrations (MICs) of diospyrin against Streptococcus pyogenes ATCC 12344 and Streptococcus pneumoniae ATCC 33400 ranged from 1.56 to 50 microg/mL. While those against Salmonella choleraesuis serotype typhi (S. typhi), ATCC 6539 and Mycobacterium chelonae ATCC 19977 were between 25 and 100 microg/mL. Isodiospyrin was more active than its racemic isomer diospyrin. The MICs against Gram-positive bacteria ranged from 0.78 to 50 microg/mL. While those against Pseudomonas aeruginosa ATCC 15443 and S. typhi ranged from 50 to 100 microg/mL. The MIC for M. chelonae was between 6.25 and 25 microg/mL. MICs were found to increase with the concentration of cells used for the inoculum. The MICs for Bacillus subtilis ATCC 6633 increased up to the highest concentration of cells tested. The same phenomenon was observed on M. chelonae, but with better effect in the latter. The kinetics of bacteria studies against both B. subtilis and M. chelonae increases with increasing concentration of Isodiospyrin tested. Two tetrameric forms of plumbagin were isolated. The naphthoquinone bisIsodiospyrin, gave MIC values between 300 and 400 micro g/mL. The second, as yet unidentified tetramer, was not active at 500 micro g/mL.
Isodiospyrin as a novel human DNA topoisomerase I inhibitor.[Pubmed:14599556]
Biochem Pharmacol. 2003 Nov 15;66(10):1981-91.
Isodiospyrin is a natural product from the plant Diospyros morrisiana, which consists of an asymmetrical 1,2-binaphthoquinone chromophore. Isodiospyrin exhibits cytotoxic activity to tumor cell lines but very little is known about its cellular target and mechanism of action. Unlike the prototypic human topoisomerase I (htopo I) poison camptothecin, Isodiospyrin does not induce htopo I-DNA covalent complexes. However, Isodiospyrin antagonizes camptothecin-induced, htopo I-mediated DNA cleavage. Binding analysis indicated that Isodiospyrin binds htopo I but not DNA. These results suggest that Isodiospyrin inhibits htopo I by direct binding to htopo I, which limits htopo I access to the DNA substrate. Furthermore, Isodiospyrin exhibits strong inhibitory effect on the kinase activity of htopo I toward splicing factor 2/alternate splicing factor in the absence of DNA. Thus, these findings have important implications on naphthoquinone and its derivatives' cellular mode of actions, i.e. these novel DNA topoisomerase I inhibitors can prevent both DNA relaxation and kinase activities of htopo I.


