(R)-3,4-DCPGCAS# 201730-10-1 |
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 201730-10-1 | SDF | Download SDF |
| PubChem ID | 6604849 | Appearance | Powder |
| Formula | C10H9NO6 | M.Wt | 239.18 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
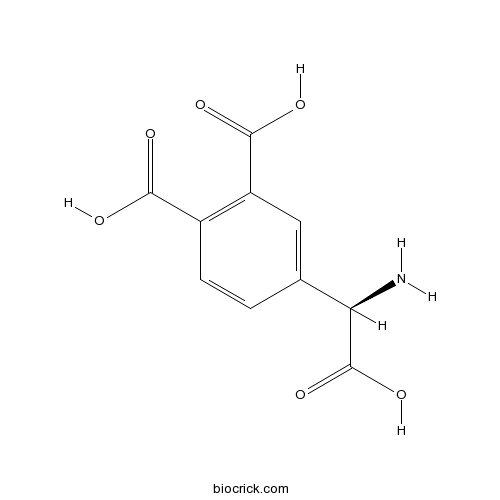
| Chemical Name | 4-[(R)-amino(carboxy)methyl]phthalic acid | ||
| SMILES | C1=CC(=C(C=C1C(C(=O)O)N)C(=O)O)C(=O)O | ||
| Standard InChIKey | IJVMOGKBEVRBPP-SSDOTTSWSA-N | ||
| Standard InChI | InChI=1S/C10H9NO6/c11-7(10(16)17)4-1-2-5(8(12)13)6(3-4)9(14)15/h1-3,7H,11H2,(H,12,13)(H,14,15)(H,16,17)/t7-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AMPA receptor antagonist with weak activity at NMDA receptors and little activity at kainate receptors. (RS)-3,4-DCPG and (S)-3,4-DCPG also available. |

(R)-3,4-DCPG Dilution Calculator

(R)-3,4-DCPG Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.181 mL | 20.9048 mL | 41.8095 mL | 83.619 mL | 104.5238 mL |
| 5 mM | 0.8362 mL | 4.181 mL | 8.3619 mL | 16.7238 mL | 20.9048 mL |
| 10 mM | 0.4181 mL | 2.0905 mL | 4.181 mL | 8.3619 mL | 10.4524 mL |
| 50 mM | 0.0836 mL | 0.4181 mL | 0.8362 mL | 1.6724 mL | 2.0905 mL |
| 100 mM | 0.0418 mL | 0.209 mL | 0.4181 mL | 0.8362 mL | 1.0452 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Triptocalline A
Catalog No.:BCN6783
CAS No.:201534-10-3
- Triptocallic acid D
Catalog No.:BCN4882
CAS No.:201534-09-0
- Fmoc-D-Pen(Trt)-OH
Catalog No.:BCC3309
CAS No.:201532-01-6
- Fmoc-Pen(Trt)-OH
Catalog No.:BCC3306
CAS No.:201531-88-6
- Deferasirox
Catalog No.:BCC3924
CAS No.:201530-41-8
- Dilazep dihydrochloride
Catalog No.:BCC6660
CAS No.:20153-98-4
- Fmoc-Asn-ol
Catalog No.:BCC2586
CAS No.:201484-12-0
- Boc-Dap(Boc)-OH.DCHA
Catalog No.:BCC2664
CAS No.:201472-68-6
- PKI 14-22 amide, myristoylated
Catalog No.:BCC8087
CAS No.:201422-03-9
- Talarozole
Catalog No.:BCC1980
CAS No.:201410-53-9
- Rutundic acid
Catalog No.:BCN5370
CAS No.:20137-37-5
- Tenofovir disoproxil
Catalog No.:BCN2178
CAS No.:201341-05-1
- (S)-3,4-DCPG
Catalog No.:BCC7012
CAS No.:201730-11-2
- Isodiospyrin
Catalog No.:BCN4883
CAS No.:20175-84-2
- Ac-Phe-OH
Catalog No.:BCC3005
CAS No.:2018-61-3
- Z-Leu-OH
Catalog No.:BCC2766
CAS No.:2018-66-8
- Tenuifolin
Catalog No.:BCN5005
CAS No.:20183-47-5
- Pisatin
Catalog No.:BCN3912
CAS No.:20186-22-5
- Magnolioside
Catalog No.:BCN2832
CAS No.:20186-29-2
- Ombuoside
Catalog No.:BCN3711
CAS No.:20188-85-6
- PTAC oxalate
Catalog No.:BCC6217
CAS No.:201939-40-4
- Epicatechin pentaacetate
Catalog No.:BCN4884
CAS No.:20194-41-6
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- Kaempferol 7-O-rhamnoside
Catalog No.:BCN6489
CAS No.:20196-89-8
Potential antipsychotic and extrapyramidal effects of (R,S)-3,4-dicarboxyphenylglycine [(R,S)-3,4-DCPG], a mixed AMPA antagonist/mGluR8 agonist.[Pubmed:15215559]
Pol J Pharmacol. 2004 May-Jun;56(3):295-304.
An involvement of glutamatergic transmission in schizophrenia has been postulated for several years. According to that view, hypofunction of NMDA receptors and a compensatory increase in glutamate release which overstimulates non-NMDA receptors contributes to psychotic symptoms. Therefore, potential antipsychotic drugs are searched for among compounds which block AMPA receptors and inhibit glutamate release. (R,S)-3,4-dicarboxyphenylglycine [(R,S)-3,4-DCPG] is a mixed antagonist of AMPA receptors and agonist of an autoreceptor, i.e. metabotropic glutamate receptor 8. The aim of the study was to look for putative antipsychotic properties of (R,S)-3,4-DCPG in the model of locomotor stimulation induced by amphetamine or phencyclidine in mice. Moreover, a risk of extrapyramidal side-effects induced by this compound was examined, as capability to induce catalepsy in the bar test and to increase the proenkephalin mRNA expression, measured autoradiographically in striatal slices by in situ hybridization. (R,S)-3,4-DCPG (80 mg/kg i.p.) decreased the amphetamine (2.5 mg/kg s.c.)-but not phencyclidine (3 mg/kg s.c.)-induced hyperactivity. That dose of (R,S)-3,4-DCPG did not decrease the spontaneous locomotor activity of mice. However, a dose of 100 mg/kg ip of that compound evoked catalepsy and enhanced the catalepsy and striatal proenkephalin mRNA expression induced by haloperidol (1-2 mg/kg i.p.). The study seems to suggest that (R,S)-3,4-DCPG may possess antipsychotic properties at doses close to those evoking extrapyramidal side-effects which speaks for its rather typical than atypical neuroleptic profile.
Anticonvulsant activity of 3,4-dicarboxyphenylglycines in DBA/2 mice.[Pubmed:11311902]
Neuropharmacology. 2001 Apr;40(5):732-5.
The 3,4-dicarboxyphenylglycines (3,4-DCPG) inhibit sound-induced seizures in DBA/2 mice with the racemate being notably more potent than either isomer (ED(50) (nmol, i.c.v.)): (RS)-3,4-DCPG (0.004; 86 mg/kg, i.p.)>>the mGlu(8) agonist (S)-3,4-DCPG (0.11)>the AMPA antagonist (R)-3,4-DCPG (0.38). A potentiation of anticonvulsant activity between AMPA and mGlu(8) receptors was confirmed by combining (R)-3,4-DCPG with the mGlu(8) agonist (RS)-4-phosphonophenylglycine. This potentiating mechanism provides a novel strategy for the treatment of epileptic seizures.
Dicarboxyphenylglycines antagonize AMPA- but not kainate-induced depolarizations in neonatal rat motoneurones.[Pubmed:9455991]
Eur J Pharmacol. 1997 Nov 5;338(2):111-6.
Ionotropic glutamate receptors have been categorized into three main groups according to the selective agonists that activate them, the N-methyl-D-aspartate (NMDA), (S)-2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propanoic acid (AMPA) and (2S,3S,4S)-3-carboxymethyl-4-isopropenylpyrrolidine-2-carboxylic acid (kainate) receptors. Both AMPA and kainate induce depolarizations in neonatal rat spinal motoneurones. However, selective antagonists capable of discriminating between the effects of these two antagonists are not widely available. As part of a search for such compounds we report the actions of (RS)-3,4-dicarboxyphenylglycine (DCPG) and (RS)-3,5-dicarboxyphenylglycine on agonist-induced motoneuronal depolarizations in the neonatal rat spinal cord preparation. In addition, the actions of (R)- and (S)-3,4-DCPG are also described. (RS)-3,4-DCPG and (RS)-3,5-DCPG antagonized AMPA-induced depolarizations (apparent Kd = 137 microM (n = 3) and 167 microM (n = 5), respectively). However, (RS)-3,5-DCPG (1 mM) potentiated responses due to kainate (n = 5) while (RS)-3,4-DCPG (1 mM) displayed weak antagonism of these responses (apparent Kd > 12 mM, n = 3). (RS)-3,4- and (RS)-3,5-DCPG at 500 microM both displayed antagonism at the NMDA receptor (apparent Kd = 472 microM and 346 microM, respectively) and a postsynaptic subgroup I metabotropic glutamate receptor activated by (1S,3R)-ACPD. The AMPA receptor antagonist activity of (RS)-3,4-DCPG was shown to reside in the (R)-enantiomer (apparent Kd = 77 microM, n = 3). The same isomer was responsible for the NMDA receptor antagonism while showing little or no antagonism of kainate-induced depolarizations (apparent Kd > 3 mM, n = 3), and a weak antagonistic effect at (1S,3R)-ACPD receptors. (S)-3,4-DCPG (500 microM) was unable to antagonize kainate-induced depolarizations, showed weak or no antagonism of NMDA- and AMPA-induced depolarizations, but antagonized (1S,3R)-ACPD-induced depolarizations. Thus (RS)-3,4-, (RS)-3,5- and (R)-3,4-DCPG demonstrate useful discrimination of responses due to AMPA and kainate, strongly suggesting that pharmacologically distinct AMPA and kainate receptors exist in motoneurones in the neonatal rat spinal cord.


