HOBt (anhydrous)Racemization inhibitor CAS# 2592-95-2 |
Quality Control & MSDS
Number of papers citing our products

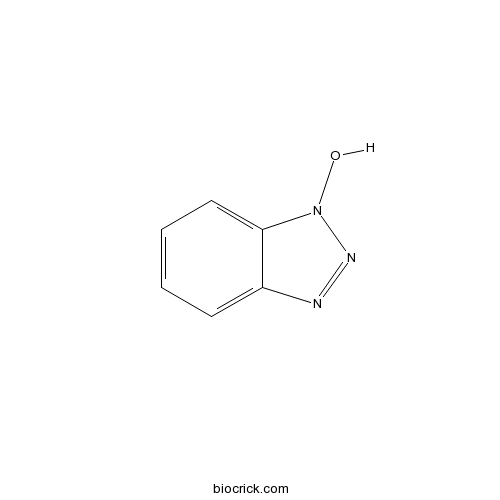
Chemical structure

3D structure
| Cas No. | 2592-95-2 | SDF | Download SDF |
| PubChem ID | 75771 | Appearance | Powder |
| Formula | C6H5N3O | M.Wt | 135.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >6.8mg/mL in DMSO | ||
| Chemical Name | 1-hydroxybenzotriazole | ||
| SMILES | C1=CC=C2C(=C1)N=NN2O | ||
| Standard InChIKey | ASOKPJOREAFHNY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H5N3O/c10-9-6-4-2-1-3-5(6)7-8-9/h1-4,10H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | HOBt is an inhibitor of racemization for peptide synthesis. | |||||
| Targets | racemization | |||||

HOBt (anhydrous) Dilution Calculator

HOBt (anhydrous) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.4019 mL | 37.0096 mL | 74.0192 mL | 148.0385 mL | 185.0481 mL |
| 5 mM | 1.4804 mL | 7.4019 mL | 14.8038 mL | 29.6077 mL | 37.0096 mL |
| 10 mM | 0.7402 mL | 3.701 mL | 7.4019 mL | 14.8038 mL | 18.5048 mL |
| 50 mM | 0.148 mL | 0.7402 mL | 1.4804 mL | 2.9608 mL | 3.701 mL |
| 100 mM | 0.074 mL | 0.3701 mL | 0.7402 mL | 1.4804 mL | 1.8505 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
HOBt was proposed as an additive to DCC to reduce racemization. Today, majority of peptide drugs up to 13-15 amino acids are synthesized using solution approach, Boc chemistry and conventional DCC/ HOBt method
- Boc-Lys(Boc)-ONp
Catalog No.:BCC3414
CAS No.:2592-19-0
- Boc-Thr-OH
Catalog No.:BCC3449
CAS No.:2592-18-9
- 3'-Hydroxydehydroaglaiastatin
Catalog No.:BCN7725
CAS No.:259143-58-3
- D-Luciferin
Catalog No.:BCC6535
CAS No.:2591-17-5
- Yunaconitoline
Catalog No.:BCN6703
CAS No.:259099-25-7
- Hoechst 33258 analog
Catalog No.:BCC1624
CAS No.:258843-62-8
- Liensinine
Catalog No.:BCN6337
CAS No.:2586-96-1
- H-D-Cys(Trt)-OH
Catalog No.:BCC2914
CAS No.:25840-82-8
- Ghrelin (rat)
Catalog No.:BCC5767
CAS No.:258338-12-4
- IEM 1925 dihydrobromide
Catalog No.:BCC7885
CAS No.:258282-23-4
- Ghrelin (human)
Catalog No.:BCC7076
CAS No.:258279-04-8
- LEP (116-130) (mouse)
Catalog No.:BCC1016
CAS No.:258276-95-8
- EDC.HCl
Catalog No.:BCC2812
CAS No.:25952-53-8
- Allura Red AC
Catalog No.:BCN2220
CAS No.:25956-17-6
- 5-Acetyl-3-chloro-10,11-dihydro-5H-dibenz[b,f]azepine
Catalog No.:BCC8726
CAS No.:25961-11-9
- 5-Hydroxy-7-methoxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1472
CAS No.:259653-54-8
- 2-Deacetyltaxuspine X
Catalog No.:BCN7375
CAS No.:259678-73-4
- T 705
Catalog No.:BCC4130
CAS No.:259793-96-9
- Hyponine D
Catalog No.:BCC8998
CAS No.:259823-31-9
- 4-Hydroxythiobenzamide
Catalog No.:BCC8709
CAS No.:25984-63-8
- Nimbolide
Catalog No.:BCN8053
CAS No.:25990-37-8
- Xylose
Catalog No.:BCC4880
CAS No.:25990-60-7
- GI 254023X
Catalog No.:BCC2374
CAS No.:260264-93-5
- Zamanic acid
Catalog No.:BCN5131
CAS No.:260393-05-3
Studies on the metabolic clearance of ciclotropium to alpha-phenylciclopentylacetic acid using a new enantiospecific metabolite assay.[Pubmed:1492851]
Arzneimittelforschung. 1992 Nov;42(11):1354-8.
Ester hydrolysis represents an important biotransformation pathway for various parasympatholytic agents. Cleavage of the ciclotropium ester bond results in the formation of alpha-phenylciclopentylacetic acid (PCA). The relevance of this metabolic route for ciclotropium bromide (HIT-PCE, CAS 85166-20-7) including its stereochemical aspects was studied in a preliminary pharmacokinetic study. An enantiospecific assay for biological material was developed that is based on chiral derivatization of PCA with N-ethyl-N'-(3-dimethylaminopropyl)carbodiimide (EDAC) and the primary amine S-FLOPA, a chiral coupling component for carboxylic acids derived from S-flunoxaprofen, followed by HPLC resolution. R-(--)-Ibuprofen was used as internal standard. From plasma or urine PCA can be extracted into n-hexane/ethanol (9:1) at pH 4 under addition of sodium chloride. Derivatization with EDAC/FLOPA was performed under addition of 1-hydroxybenzotriazole in anhydrous dichloromethane that contained trace amounts of pyridine (ambient temperature; 2 h reaction time). The chromatographic separation was performed on a silica gel stationary phase (Zorbax Sil) using n-hexane-chloroform-ethanol (100:10:1, by vol.) as mobile phase (flow rate, 2 ml/min; fluorescence-detection, 305/355 nm; elution order of the derivatives, (-) before (+)). Limit of quantification was 1.0 ng/ml for plasma and 10 ng/ml for urine. In the pharmacokinetic study in two healthy volunteers who received a single i.v. dose of 10 mg ciclotropium race-mate the PCA concentrations in plasma were below the detection limit, but approx. 1.5% of the administered dose were excreted into urine as the respective glucuronides.(ABSTRACT TRUNCATED AT 250 WORDS)
Characterization and catalytic property of surfactant-laccase complex in organic media.[Pubmed:10933832]
Biotechnol Prog. 2000 Jul-Aug;16(4):583-8.
The oxidation of o-phenylenediamine catalyzed in anhydrous organic solvents by surfactant-laccase complex was investigated. The complex was prepared by utilizing a novel preparation technique in water-in-oil (W/O) emulsions. The surfactant-laccase complex effectively catalyzed the oxidation reaction in various dry organic solvents, while laccase, lyophilized from an aqueous buffer solution in which its activity was optimized, exhibited no catalytic activity in nonaqueous media. To optimize the preparation and reaction conditions for the surfactant-enzyme complexes, we examined the effects of pH in the water pool of W/O emulsions, the concentration of enzyme and surfactant at the preparation stage, and the nature of organic solvents at the reaction stage on the laccase activity in organic media. Surfactant-laccase complex showed a strong pH-dependent catalytic activity in organic media. Its optimum activity was obtained when the complex was prepared at a pH of about 3. Interestingly, native laccase in an aqueous buffer solution exhibited an optimum activity at the same pH of 3. The optimum preparation conditions of surfactant-laccase complex were [laccase] = 0.8 mg/mL and [surfactant] = 10 mM, and the complex showed the highest catalytic activity in toluene among nine anhydrous organic solvents. The effect of a cosolubilized mediator (1-hydroxybenzotriazole (HBT)) on the reaction was also investigated. The addition of HBT at the preparation stage of the enzyme complex did not accelerate the catalytic reaction because HBT was converted to an inactive benzotriazole (BT) by laccase. However, the addition of HBT at the reaction stage enhanced the catalytic performance by a factor of five compared to that without HBT.
Synthesis and pharmacological properties of [1-L-penicillamine,4-L-leucine]oxytocin.[Pubmed:1159679]
J Med Chem. 1975 Oct;18(10):1020-2.
For the synthesis of [1-L-penicillamine,4-L-leucine]oxytocin (2), Z-Tyr(Bzl)-Ile-Leu-Asn-Cys(Bzl)-Pro-Leu-Gly-NH2 was treated with anhydrous HBr, and the resulting partially deprotected octapeptide was coupled with Z-penicillamine(Bzl) in a condensation reaction mediated by dicyclohexylcarbodiimide and 1-hydroxybenzotriazole. The protected nonapeptide Z-penicillamine(Bzl)-Tyr-Ile-Leu-Asn-Cys(Bzl)-Pro-Leu-Gly-NH2 was treated with Na in NH3 and the resulting disulfhydryl compound was subjected to oxidative cyclization in H2O-CH3OH with ICH2CH2I, Purification of 2 was effected by partition chromatography and gel filtration. The analog possesses antioxytocic and antiavian vasodepressor pA2 values of 6.77 and 7.21, respectively, and has no antipressor or anti-ADH activity. Its biological activity spectrum is qualitatively identical with that of [1-penicillamine]oxytocin. In contrast to the marked natriuretic-diuretic and anti-antidiuretic activity of [Leu4]oxytocin, 2 exhibits none of these effects on the rat kidney.
Unexpected racemization of proline or hydroxy-proline phenacyl ester during coupling reactions with Boc-amino acids.[Pubmed:1446968]
Int J Pept Protein Res. 1992 Aug;40(2):114-8.
When L-proline or O-benzyl-trans-4-hydroxy-L-proline phenacyl ester was coupled with Boc-amino acids in dimethylformamide using water-soluble carbodiimide (WSCI) in the presence of anhydrous 1-hydroxybenzotriazole (HOBt) as coupling reagents, extensive racemization was observed at the C alpha of the proline or hydroxy-proline residue. The extent of racemization was measured by HPLC after the coupling with Boc-L-Leu-OH in the presence or absence of HOBt. The extent of racemization increased when HOBt was added to the reaction mixture, but greatly decreased when it was not, indicating that HOBt was needed for inducing racemization. Almost no racemization was observed when the coupling reaction was carried out by the mixed anhydride procedure in tetrahydrofuran or by the carbodiimide method in dichloromethane without using HOBt. In the case of coupling reactions with ordinary L-amino acid phenacyl esters, no racemization was observed. Examination of some model systems yielded sufficient evidence to prove that HOBt is an efficient catalyst for racemizing proline or hydroxy-proline phenacyl ester not only in the stage of cyclic intermediate formation but also in the opening of the ring structure. Thus, the racemization reaction was found to be closely related to the formation of the cyclic carbinol-amine derivative.
Pharmacokinetic studies with the lipid-regulating agent beclobrate: enantiospecific assay for beclobric acid using a new fluorescent chiral coupling component (S-FLOPA).[Pubmed:2039683]
Chirality. 1991;3(1):35-42.
The major biotransformation pathway for the chiral lipid-regulating agent beclobrate is conversion to the corresponding carboxylic acid, which is then metabolized to the acyl glucuronide. An enantiospecific assay for biological material was developed that is based on chiral derivatization with N-ethyl-N'-(3-dimethylaminopropyl)carbodiimide (EDAC) and the primary amine S-FLOPA, a new chiral coupling component for carboxylic acids derived from the 2-arylpropionic acid S-flunoxaprofen. Conversion of beclobric acid to the acyl chloride prior to coupling with the amine is also feasible. From plasma or urine beclobric acid was extracted into n-hexane/ethanol (9:1) at pH 4 after addition of sodium chloride. Clofibric acid was used as internal standard. Derivatization with EDAC/FLOPA was performed under addition of 1-hydroxybenzotriazole in anhydrous dichloromethane containing trace amounts of pyridine (ambient temperature/2 h reaction time). The chromatographic separation was performed on a silica gel stationary phase (Zorbax Sil) using n-hexane-chloroform-ethanol (100:10:0.75, by vol) as mobile phase [flow rate, 2 ml/min; fluorescence detection, 305/355 nm; elution order of the derivatives, (-) before (+)]. Coefficients of variation were between 1.3 and 9.3% for both plasma and urine. Limit of quantification was 20-25 ng/ml for plasma based on a sample volume of 0.2 ml. Application of the assay in a pilot pharmacokinetic study showed significant differences between the kinetics of the two enantiomers. In plasma and urine, the concentrations of the dextrorotatory enantiomer exceeded those of the levorotatory enantiomer significantly.


