EDC.HClWater soluble condensing reagent CAS# 25952-53-8 |
- Ruscogenin
Catalog No.:BCN6287
CAS No.:472-11-7
- Pectolinarigenin
Catalog No.:BCN5813
CAS No.:520-12-7
- Dauricine
Catalog No.:BCN4977
CAS No.:524-17-4
- Oroxin A
Catalog No.:BCN1202
CAS No.:57396-78-8
- Triptophenolide
Catalog No.:BCN2546
CAS No.:74285-86-2
- Wilforlide A
Catalog No.:BCN4383
CAS No.:84104-71-2
Quality Control & MSDS
Number of papers citing our products

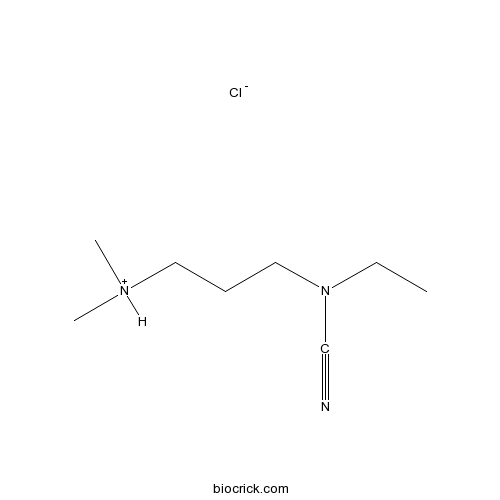
Chemical structure

3D structure
| Cas No. | 25952-53-8 | SDF | Download SDF |
| PubChem ID | 33250 | Appearance | Powder |
| Formula | C8H18ClN3 | M.Wt | 191.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
| Chemical Name | 3-[cyano(ethyl)amino]propyl-dimethylazanium;chloride | ||
| SMILES | CCN(CCC[NH+](C)C)C#N.[Cl-] | ||
| Standard InChIKey | FDXPUDRRFDHONO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H17N3.ClH/c1-4-11(8-9)7-5-6-10(2)3;/h4-7H2,1-3H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

EDC.HCl Dilution Calculator

EDC.HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2165 mL | 26.0824 mL | 52.1648 mL | 104.3297 mL | 130.4121 mL |
| 5 mM | 1.0433 mL | 5.2165 mL | 10.433 mL | 20.8659 mL | 26.0824 mL |
| 10 mM | 0.5216 mL | 2.6082 mL | 5.2165 mL | 10.433 mL | 13.0412 mL |
| 50 mM | 0.1043 mL | 0.5216 mL | 1.0433 mL | 2.0866 mL | 2.6082 mL |
| 100 mM | 0.0522 mL | 0.2608 mL | 0.5216 mL | 1.0433 mL | 1.3041 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Not available.
EDC.HCl, which is commonly regarded as carbodiimide reagents, serves as a very useful tool for the formation of amide bonds (peptide bonds) in an aqueous medium. As one of the carbodiimide reagents, it is also effectively applied in anhydroxydation, polynucleotide synthesis, esterification and lactonization.In addition, EDC.HCl is also widely used as water soluble condensing reagent. [1]
In vitro: Carbodiimide-mediated coupling method was one of the most traditional approaches for peptide-bond formation. With the aim to synthesis target compounds, carbodiimide reagents were added after thoroughly mixing of a carboxylic acid and an amine. After the reaction was accomplished, the carbodiimide reagent was then changed into its corresponding urea. [2] Recently, a simple and fast detection method using spectrophotometric flow injection analysis was developed. This method was based on the reaction between EDC.HCl and pyridine in acidic aqueous solution. After reaction at 40C for a while, the absorbance was measured at 400 nm. The calibration curve demonstrated a good linearity from 0 to 10% of EDC·HCl solutions with the following regression equation: y = 3.15 × 104x (x, % concentration of EDC·HCl; y, peak area). This approach served to monitoring the concentration of EDC·HCl after its reaction in water. [1]
In vivo: So far, no in vivo data has been reported.
Clinical trial: So far, no clinical trial has been conducted.
References:
[1]Kunihiko Seno, Kazuki Matumura, Mitsuko Oshima, and Shoji Motomizu. Spectrophotometric determination of 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride by flow injection analysis. Anal Sci. 2008 Apr; 24: 505-8.
[2] Jad YE, Khattab SN, Beatriz G. Torre DL, Govender T, Kruger HG, Faham AE and Albericio F. EDC·HCl and potassium salts of oxyma and oxyma-B as superior coupling cocktails for peptide synthesis. Eur J Org Chem. 2015 May; 2015(14): 3116-20.
- HOBt (anhydrous)
Catalog No.:BCC2816
CAS No.:2592-95-2
- Boc-Lys(Boc)-ONp
Catalog No.:BCC3414
CAS No.:2592-19-0
- Boc-Thr-OH
Catalog No.:BCC3449
CAS No.:2592-18-9
- 3'-Hydroxydehydroaglaiastatin
Catalog No.:BCN7725
CAS No.:259143-58-3
- D-Luciferin
Catalog No.:BCC6535
CAS No.:2591-17-5
- Yunaconitoline
Catalog No.:BCN6703
CAS No.:259099-25-7
- Hoechst 33258 analog
Catalog No.:BCC1624
CAS No.:258843-62-8
- Liensinine
Catalog No.:BCN6337
CAS No.:2586-96-1
- H-D-Cys(Trt)-OH
Catalog No.:BCC2914
CAS No.:25840-82-8
- Ghrelin (rat)
Catalog No.:BCC5767
CAS No.:258338-12-4
- IEM 1925 dihydrobromide
Catalog No.:BCC7885
CAS No.:258282-23-4
- Ghrelin (human)
Catalog No.:BCC7076
CAS No.:258279-04-8
- Allura Red AC
Catalog No.:BCN2220
CAS No.:25956-17-6
- 5-Acetyl-3-chloro-10,11-dihydro-5H-dibenz[b,f]azepine
Catalog No.:BCC8726
CAS No.:25961-11-9
- 5-Hydroxy-7-methoxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1472
CAS No.:259653-54-8
- 2-Deacetyltaxuspine X
Catalog No.:BCN7375
CAS No.:259678-73-4
- T 705
Catalog No.:BCC4130
CAS No.:259793-96-9
- Hyponine D
Catalog No.:BCC8998
CAS No.:259823-31-9
- 4-Hydroxythiobenzamide
Catalog No.:BCC8709
CAS No.:25984-63-8
- Nimbolide
Catalog No.:BCN8053
CAS No.:25990-37-8
- Xylose
Catalog No.:BCC4880
CAS No.:25990-60-7
- GI 254023X
Catalog No.:BCC2374
CAS No.:260264-93-5
- Zamanic acid
Catalog No.:BCN5131
CAS No.:260393-05-3
- Dihydroisotanshinone II
Catalog No.:BCN5132
CAS No.:260397-58-8
3,6-O-[N-(2-Aminoethyl)-acetamide-yl]-chitosan exerts antibacterial activity by a membrane damage mechanism.[Pubmed:27261735]
Carbohydr Polym. 2016 Sep 20;149:102-11.
A novel chitosan derivative, 3,6-O-[N-(2-aminoethyl)-acetamide-yl]-chitosan (AACS), was successfully prepared to improve water solubility and antibacterial activity of chitosan. AACS had good antibacterial activity, with minimum inhibitory concentrations of 0.25mg/mL, against Escherichia coli and Staphylococcus aureus. Cell membrane integrity, electric conductivity and NPN uptake tests showed that AACS caused quickly increasing the release of intracellular nucleic acids, the uptake of NPN, and the electric conductivity by damaging membrane integrity. On the other hand, hydrophobicity, cell viability and SDS-PAGE experiments indicated that AACS was able to reduce the surface hydrophobicity, the cell viability and the intracellular proteins through increasing membrane permeability. SEM observation further confirmed that AACS could kill bacteria via disrupting their membranes. All results above verified that AACS mainly exerted antibacterial activity by a membrane damage mechanism, and it was expected to be a new food preservative.
Approach toward the Understanding of Coupling Mechanism for EDC Reagent in Solvent-Free Mechanosynthesis.[Pubmed:28937766]
Org Lett. 2017 Oct 6;19(19):5360-5363.
A unique approach in mechanosynthesis, joining solid-state NMR spectroscopy, X-ray crystallography, and theoretical calculations, is employed for the first time to study the mechanism of the formation of the C-N amide bond using EDC.HCl as a coupling reagent. It has been proved that EDC.HCl, which in the crystal lattice exists exclusively in the cyclic form (X-ray data), easily undergoes transformation to a pseudocyclic stable intermediate in reaction with carboxylic acid forming a low-melt phase (differential scanning calorimetry, solid-state NMR). The obtained intermediate is reactive and can be further used for synthesis of amides in reaction with appropriate amines.
Glucosamine-anchored doxorubicin-loaded targeted nano-niosomes: pharmacokinetic, toxicity and pharmacodynamic evaluation.[Pubmed:26878084]
J Drug Target. 2016 Sep;24(8):730-43.
BACKGROUND: Efficacy of anticancer drug is limited due to non-selectivity and toxicities allied with the drug; therefore the heart of the present work is to formulate drug delivery systems targeted selectively towards cancer cells with minimal toxicity to normal cells. PURPOSE: Targeted drug delivery system of doxorubicin (DOX)-loaded niosomes using synthesized N-lauryl glucosamine (NLG) as a targeting ligand. METHODS: NLG-anchored DOX niosomes were developed using ethanol injection method. RESULTS: Developed niosomes had particle size <150 nm and high entrapment efficiency approximately 90%. In vivo pharmacokinetics exhibited long circulating nature of targeted niosomes with improved bioavailability, which significantly reduced CL and Vd than DOX solution and non-targeted niosomes (35 fold and 2.5 fold, respectively). Tissue-distribution study and enzymatic assays revealed higher concentration of DOX solution in heart while no toxicity to major organs with developed targeted niosomes was observed. Solid skin melanoma tumor model in mice manifested the commendable targeting potential of targeted niosomes with significant reduction in tumor volume and high % survival rate without drop in body weight in comparison with DOX solution and non-targeted niosomes of DOX. CONCLUSION: The glucosamine-anchored DOX-loaded targeted niosomes showed its potential in cancer targeted drug therapy with reduced toxicity. Abbreviations ALT alanine transaminase CL clearance CPK creatinine phosphokinase DOX doxorubicin EDC.HCl ethyl carbidimide hydrochloride GLUT glucose transporter GSH glutathione S-transferase LDH lactate dehydrogenase LHRH luteinizing hormone-releasing hormone MDA malonaldehyde NHS N-hydroxy succinimide NLG N-lauryl glucosamine NTAR DoxNio non-targeted doxorubicin niosomes PBS phosphate buffer saline RGD argynyl glycyl aspartic acid SGOT serum glutamate oxaloacetate transaminase SGPT serum glutamate pyruvate transaminase SOD superoxide dismutase TAR DoxNio targeted doxorubicin niosomes Vd volume of distribution.
[Rapid detection of diethylstilbestrol using a quartz crystal microbalance with gold nanoparticals amplification].[Pubmed:26957248]
Zhonghua Yu Fang Yi Xue Za Zhi. 2016 Mar;50(3):270-3.
OBJECTIVE: To develop a quartz crystal microbalance (QCM) immunosensor with high sensitivity and selectivity for the rapid detection of diethylstilbestrol. METHODS: Dextran was used as reducing agent for preparing gold nanoparticles (AuNPs) with the size of 40 nm. The AuNPs were coupled with anti-DES antibody after amination. A monolayer was generated after immersing the quartz crystal into the solution of 5 mmol/L 11-mercaptoundecanoic acid(MUA) for 16 hours. After the monolayer was activated by 1-ethyl-3-(3-dimethylaminopropry) carbodiimide hydrochloride (EDC.HCl) and N-hydrosuccinimide (NHS), 20 mul of 2.2 mg/ml DES-HS-BSA was dropped onto the surface of crystal to prepare a sensitive membrane which can recognize DES specifically. Then, 50 mul of 1 mol/L ethanolamine (pH 8.5) was used to seal the carboxylic groups to make the sensitive membrane which could identify DES specifically. QCM immunosensor was used as detection platform to optimize the reaction conditions. Under the optimized conditions, 10 mul of 28 mug/ml AuNPs-antibody was mixed with 10 mul of 0.03-2.5 mug/ml DES, and the mixture was added on the sensitive membrane. QCM immunosensor was used to detect the signals and the standard curve was obtained at the same time. The detection limit was calculated based on the standard curve. The specificity was evaluated by testing DES and its analogues with the same concentration. RESULTS: The optimized concentration for the immobilization of DES-HS-BSA on the surface of QCM was 2.2 mg/ml. The optimized concentration for coupling anti-DES antibody with AuNPs was 7 mug/ml and 15 nmol/L, respectively. The optimized concentration of AuNPs-antibody was 14 mug/ml. The logarithm of DES concentration was proportional to the frequency shift in the range of 0.16-500 ng/ml, Deltaf=-24.170 lgCDES+69.71, R(2)=0.998. The detection limit of this method was 0.13 ng/ml. DES analogues could not influence the detection of DES obviously, so the sensor had good specificity. CONCLUSION: The quartz crystal microbalance immunosensor with gold nanoparticals amplification could detect DES sensitively and rapidly.
Synthesis of antimicrobial glucosamides as bacterial quorum sensing mechanism inhibitors.[Pubmed:28049617]
Bioorg Med Chem. 2017 Feb 1;25(3):1183-1194.
Bacteria communicate with one another and regulate their pathogenicity through a phenomenon known as quorum sensing (QS). When the bacterial colony reaches a threshold density, the QS system induces the production of virulence factors and the formation of biofilms, a powerful defence system against the host's immune responses. The glucosamine monomer has been shown to disrupt the bacterial QS system by inhibiting autoinducer (AI) signalling molecules such as the acyl-homoserine lactones (AHLs). In this study, the synthesis of acetoxy-glucosamides 8, hydroxy-glucosamides 9 and 3-oxo-glucosamides 12 was performed via the 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCl) and N,N'-dicyclohexylcarbodiimide (DCC) coupling methods. All of the synthesized compounds were tested against two bacterial strains, P. aeruginosa MH602 (LasI/R-type QS) and E. coli MT102 (LuxI/R-type QS), for QS inhibitory activity. The most active compound 9b showed 79.1% QS inhibition against P. aeruginosa MH602 and 98.4% against E. coli MT102, while compound 12b showed 64.5% inhibition against P. aeruginosa MH602 and 88.1% against E. coli MT102 strain at 2mM concentration. The ability of the compounds to inhibit the production of the virulence factor pyocyanin and biofilm formation in the P. aeruginosa (PA14) strain was also examined. Finally, computational docking studies were performed with the LasR receptor protein.


