GanaxolonePotent, positive allosteric modulator of GABAA receptors CAS# 38398-32-2 |
- DMXAA (Vadimezan)
Catalog No.:BCC3644
CAS No.:117570-53-3
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
- ABT-737
Catalog No.:BCC3613
CAS No.:852808-04-9
- TW-37
Catalog No.:BCC2257
CAS No.:877877-35-5
Quality Control & MSDS
Number of papers citing our products

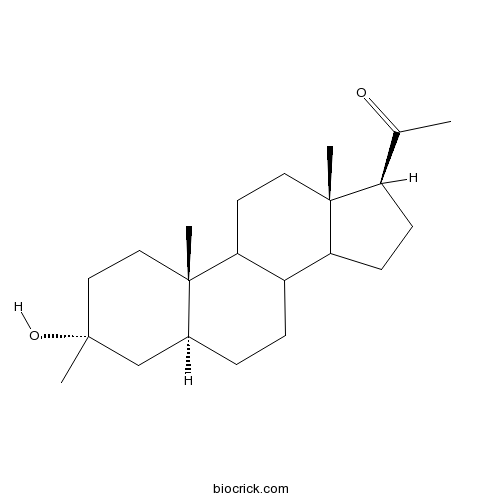
Chemical structure

3D structure
| Cas No. | 38398-32-2 | SDF | Download SDF |
| PubChem ID | 38022 | Appearance | Powder |
| Formula | C22H36O2 | M.Wt | 332.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CCD 1042 | ||
| Solubility | Soluble to 10 mM in DMSO with gentle warming and to 20 mM in ethanol | ||
| Chemical Name | 1-[(3R,5S,10S,13S,17S)-3-hydroxy-3,10,13-trimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]ethanone | ||
| SMILES | CC(=O)C1CCC2C1(CCC3C2CCC4C3(CCC(C4)(C)O)C)C | ||
| Standard InChIKey | PGTVWKLGGCQMBR-XYZZJYPZSA-N | ||
| Standard InChI | InChI=1S/C22H36O2/c1-14(23)17-7-8-18-16-6-5-15-13-20(2,24)11-12-21(15,3)19(16)9-10-22(17,18)4/h15-19,24H,5-13H2,1-4H3/t15-,16?,17+,18?,19?,20+,21-,22+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent positive allosteric modulator of GABAA receptors. Enhances GABA-evoked chloride currents in Xenopus oocytes expressing GABAA receptors (EC50 values are 94, 122 and 213 nM for α2β1γ2L, α3β1γ2L and α1β1γ2L receptors respectively). Exerts anticonvulsive effects in a broad range of animal seizure models. |

Ganaxolone Dilution Calculator

Ganaxolone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0072 mL | 15.0362 mL | 30.0725 mL | 60.1449 mL | 75.1812 mL |
| 5 mM | 0.6014 mL | 3.0072 mL | 6.0145 mL | 12.029 mL | 15.0362 mL |
| 10 mM | 0.3007 mL | 1.5036 mL | 3.0072 mL | 6.0145 mL | 7.5181 mL |
| 50 mM | 0.0601 mL | 0.3007 mL | 0.6014 mL | 1.2029 mL | 1.5036 mL |
| 100 mM | 0.0301 mL | 0.1504 mL | 0.3007 mL | 0.6014 mL | 0.7518 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Caudatin
Catalog No.:BCN5810
CAS No.:38395-02-7
- NSC 663284
Catalog No.:BCC7199
CAS No.:383907-43-5
- 3',4'-Anhydrovinblastine
Catalog No.:BCN2392
CAS No.:38390-45-3
- Taxinine
Catalog No.:BCN6944
CAS No.:3835-52-7
- Neuropeptide W-23 (human)
Catalog No.:BCC5961
CAS No.:383415-79-0
- UC 112
Catalog No.:BCC8042
CAS No.:383392-66-3
- Homovanillic Acid Sulfate
Catalog No.:BCN2226
CAS No.:38339-06-9
- 3-O-beta-D-Glucopyranosylplatycodigenin
Catalog No.:BCN7832
CAS No.:38337-25-6
- YE 120
Catalog No.:BCC6188
CAS No.:383124-82-1
- Minoxidil
Catalog No.:BCC4297
CAS No.:38304-91-5
- Naringenin trimethyl ether
Catalog No.:BCN5437
CAS No.:38302-15-7
- Burchellin
Catalog No.:BCN6676
CAS No.:38276-59-4
- Aloenin
Catalog No.:BCN8438
CAS No.:38412-46-3
- Altechromone A
Catalog No.:BCN7422
CAS No.:38412-47-4
- 3,4,4',7-Tetrahydroxyflavan
Catalog No.:BCN5438
CAS No.:38412-82-7
- Sclareol glycol
Catalog No.:BCN7007
CAS No.:38419-75-9
- H-Lys-OEt .2HCl
Catalog No.:BCC2980
CAS No.:3844-53-9
- Asatone
Catalog No.:BCN7761
CAS No.:38451-63-7
- Deacetyleupaserrin
Catalog No.:BCN7228
CAS No.:38456-39-2
- Eucannabinolide
Catalog No.:BCN7221
CAS No.:38458-58-1
- Crotafoline
Catalog No.:BCN2075
CAS No.:38494-87-0
- Tarafenacin
Catalog No.:BCC4147
CAS No.:385367-47-5
- Daphnicyclidin D
Catalog No.:BCN7081
CAS No.:385384-24-7
- Daphnicyclidin F
Catalog No.:BCN6400
CAS No.:385384-26-9
Effects of the synthetic neurosteroid ganaxolone on seizure activity and behavioral deficits in an Angelman syndrome mouse model.[Pubmed:27986596]
Neuropharmacology. 2017 Apr;116:142-150.
Angelman syndrome (AS) is a rare neurogenetic disorder characterized by severe developmental delay, motor impairments, and epilepsy. GABAergic dysfunction is believed to contribute to many of the phenotypic deficits seen in AS. We hypothesized that restoration of inhibitory tone mediated by extrasynaptic GABAA receptors could provide therapeutic benefit. Here, we report that Ganaxolone, a synthetic neurosteroid that acts as a positive allosteric modulator of synaptic and extrasynaptic GABAA receptors, was anxiolytic, anticonvulsant, and improved motor deficits in the Ube3a-deficient mouse model of AS when administered by implanted mini-pump for 3 days or 4 weeks. Treatment for 4 weeks also led to recovery of spatial working memory and hippocampal synaptic plasticity deficits. This study demonstrates that Ganaxolone ameliorates many of the behavioral abnormalities in the adult AS mouse, and tolerance did not occur to the therapeutic effects of the drug. The results support clinical studies to investigate Ganaxolone as a symptomatic treatment for AS.
Evaluation of the neuroactive steroid ganaxolone on social and repetitive behaviors in the BTBR mouse model of autism.[Pubmed:26525567]
Psychopharmacology (Berl). 2016 Jan;233(2):309-23.
RATIONALE: Abnormalities in excitatory/inhibitory neurotransmission are hypothesized to contribute to autism spectrum disorder (ASD) etiology. BTBR T (+) Itpr3 (tf) /J (BTBR), an inbred mouse strain, displays social deficits and repetitive self-grooming, offering face validity to ASD diagnostic symptoms. Reduced GABAergic neurotransmission in BTBR suggests that GABAA receptor positive allosteric modulators (PAMs) could improve ASD-relevant BTBR phenotypes. The neuroactive steroid Ganaxolone acts as a PAM, displaying anticonvulsant properties in rodent epilepsy models and an anxiolytic-like profile in the elevated plus-maze. OBJECTIVES: We evaluated Ganaxolone in BTBR and C57BL/6J mice in standardized assays for sociability and repetitive behaviors. Open field and anxiety-related behaviors were tested as internal controls and for comparison with the existing neuroactive steroid literature. RESULTS: Ganaxolone improved aspects of social approach and reciprocal social interactions in BTBR, with no effect on repetitive self-grooming, and no detrimental effects in C57BL/6J. Ganaxolone increased overall exploratory activity in BTBR and C57BL/6J in the open field, social approach, and elevated plus-maze, introducing a confound for the interpretation of social improvements. Allopregnanolone and diazepam similarly increased total entries in the elevated plus-maze, indicating that behavioral activation may be a general property of GABAA receptor PAMs in these strains. CONCLUSIONS: Ganaxolone shows promise for improving sociability. In addition, Ganaxolone, as well as other GABAA receptor PAMs, enhanced overall BTBR activity. The translational implications of specific sociability improvements and nonspecific behavioral activation by Ganaxolone in the BTBR model remain to be determined. Future studies to explore whether PAMs provide a novel profile with unique benefits for ASD treatment will be worthwhile.
Effect of nucleus accumbens shell infusions of ganaxolone or gaboxadol on ethanol consumption in mice.[Pubmed:25342197]
Psychopharmacology (Berl). 2015 Apr;232(8):1415-26.
RATIONALE: Allopregnanolone (ALLO) is an endogenous neuroactive steroid thought to alter the reinforcement value of alcohol (ethanol) due to its actions as a positive modulator of the GABAA receptor (GABAAR). Extrasynaptic GABAARs may be a particularly sensitive target of ethanol and neuroactive steroids. Previous work showed that systemic injections of an ALLO analog, Ganaxolone (GAN), or an extrasynaptic GABAAR agonist (gaboxadol; THIP) decreased ethanol intake in male mice with limited access to ethanol. OBJECTIVES: The present studies tested whether activation of GABAARs in the nucleus accumbens (NAc) shell by GAN or THIP was sufficient to reduce ethanol intake. C57BL/6J male mice had 2-h access to 10 % ethanol (10E) and water, and 10E intake was measured following site-specific infusions of GAN or THIP. RESULTS: Decreases in limited-access 10E consumption were observed following site-specific bilateral infusions of either drug into the NAc shell. Significant changes in intake were absent when the drugs were infused in a region dorsal to the target site (GAN) or into the lateral ventricle (THIP). Locomotor data confirmed that the decreases in intake were not due to a sedative effect of the drugs. CONCLUSIONS: These data demonstrate the sufficiency of GABAAR activation by a positive allosteric modulator or an agonist with selectivity for extrasynaptic GABAARs to decrease ethanol consumption in mice. Importantly, more refined GABAAR-active targets that decrease ethanol intake may enhance our understanding and ability to treat alcohol use disorders.
Randomized, double-blind, placebo-controlled phase 2 study of ganaxolone as add-on therapy in adults with uncontrolled partial-onset seizures.[Pubmed:28230252]
Epilepsia. 2017 Apr;58(4):558-564.
OBJECTIVE: To evaluate the efficacy and safety of Ganaxolone as adjunctive therapy in adults with uncontrolled partial-onset seizures despite taking up to three concomitant antiepileptic drugs (AEDs). METHODS: Adults aged 18-69 years and refractory to conventional AEDs were enrolled in a multicenter, double-blind, placebo-controlled trial. After an 8-week baseline period, patients were randomized 2:1 to Ganaxolone 1,500 mg/day or placebo for a 10-week treatment period (2-week forced titration and 8-week maintenance) followed by either tapering or entry into an open-label extension study. The primary endpoint was mean weekly seizure frequency. Secondary endpoints included the proportion of patients experiencing >/=50% reduction in seizure frequency (responder rate), percent change in mean weekly seizure frequency, seizure-free days, and quality of life. Safety and tolerability assessments included adverse events (AEs), treatment discontinuation, and clinical laboratory evaluations. Efficacy analyses were performed on the intent-to-treat population. RESULTS: Of 147 randomized patients (98 Ganaxolone, 49 placebo), 131 completed the study; 95% of participants titrated up to 1,500 mg/day and 78% maintained this dose. From baseline to endpoint, mean weekly seizure frequency decreased with Ganaxolone (6.5-5.2) versus placebo (9.2-10.8), representing an 11.4% decrease versus placebo (p = 0.0489, analysis of covariance [ANCOVA]). Mean percent change from baseline was -17.6% with Ganaxolone versus 2.0% with placebo (p = 0.0144, Kruskal-Wallis test). Responder rates were 24% with Ganaxolone versus 15% with placebo (p = 0.19). Discontinuation due to adverse events was similar with Ganaxolone (7.1%) and placebo (6.1%). Common adverse events were mild to moderate in severity and included dizziness (16.3% vs. 8.2%), fatigue (16.3% vs. 8.2%), and somnolence (13.3% vs. 2.0%). SIGNIFICANCE: Ganaxolone 1,500 mg/day reduced partial-onset seizure frequency and was generally safe and well tolerated in this phase 2 study. These results support continued development of Ganaxolone for adult patients with refractory partial-onset seizures.
Effect of ganaxolone in a rodent model of cerebral hematoma.[Pubmed:10625734]
Stroke. 2000 Jan;31(1):169-75.
BACKGROUND AND PURPOSE: Therapy with gamma-aminobutyric acid (GABA) agonists appears to improve outcome after experimental hematoma but with unacceptable side effects. We looked to synthetic GABA agonists, or positive GABA modulators, widely developed as anticonvulsants and anxiolytics, to find compounds that may be effective. Ganaxolone is a synthetic neuroactive steroid that positively modulates GABA. We sought to determine whether Ganaxolone was beneficial using a model of intracerebral hematoma. METHODS: We stereotaxically injected varying doses of bacterial collagenase into the caudate nucleus of rats to induce blood-brain barrier failure and hematoma formation. Four hours later, we administered intravenously 15 or 30 mg/kg Ganaxolone (n=23 each group), 20 mg/kg pregnanolone (n=21), or vehicle (n=30). Forty-eight hours after collagenase injection, we rated each animal using a standard rodent neurological examination. The ratings were compared with the amounts of injected collagenase using the quantal bioassay procedure. Other sets of animals were tested later for visuospatial learning. Brains were then prepared for histomorphometry, and brain volumes were estimated. RESULTS: We found that Ganaxolone 30 mg/kg significantly increased the ED(50) in the bioassay, for a potency ratio of 1.8+/-0.41 compared with vehicle (P<0.05). Ganaxolone 15 mg/kg and pregnanolone did not affect neurological outcome. Ganaxolone 30 mg/kg did not clearly improve visuospatial learning several weeks after hemorrhage. Ganaxolone exhibited a weak effect on cerebral volumes 48 hours after stroke, but 3 months after hemorrhage no such effect could be detected. CONCLUSIONS: Ganaxolone improves neurological outcome 48 hours after intracerebral hematoma but not visuospatial learning several weeks after intracerebral hematoma. Histological evidence of damage was reduced at 48 hours but not at 3 months.
Modification of behavioral effects of drugs in mice by neuroactive steroids.[Pubmed:10928304]
Psychopharmacology (Berl). 2000 Mar;148(4):336-43.
RATIONALE: Neuroactive steroids represent a novel class of potential therapeutic agents (epilepsy, anxiety, migraine, drug dependence) thought to act through positive allosteric modulation of the GABA(A) receptor. A synthetically derived neuroactive steroid, Ganaxolone (3alphahydroxy-3beta-methyl-5alpha-pregnan-20-one), is in phase-II clinical trials for epilepsy. Unlike traditional anticonvulsants such as diazepam and phenobarbital, Ganaxolone shows equipotent suppression of both the seizure activity and the behavioral effects of pentylenetetrazol (PTZ) administration. OBJECTIVES: The present study explored possible reversal by Ganaxolone and related neuroactive steroids of some behavioral effects of additional pharmacological challenges. METHODS: Direct behavioral observation and photocell-counted locomotor activity of male, Swiss-Webster mice were made with various compounds alone and in conjunction with Ganaxolone. RESULTS: Ganaxolone both prevented and reversed PTZ-induced locomotor depression in mice. Further, Ganaxolone reversed the locomotor depression induced by other convulsant/anxiogenic stimuli: bicuculline, picrotoxin and, to a lesser extent, yohimbine. Ganaxolone failed to reverse the locomotor stimulation induced by cocaine, methamphetamine, dizocilpine, and phencyclidine. In addition to Ganaxolone, the endogenous neuroactive steroids allopregnanolone and pregnanolone and the synthetic neuroactive steroid Co 2-1068 also reversed observed behaviors and locomotor depression induced by PTZ. CONCLUSIONS: The present findings support the unique pharmacological effects of neuroactive steroids as a novel class of positive allosteric modulators of GABA.
Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor.[Pubmed:9067315]
J Pharmacol Exp Ther. 1997 Mar;280(3):1284-95.
Ganaxolone (CCD 1042) is a 3beta-methyl-substituted analog of the endogenous neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one. Ganaxolone inhibited binding of the gamma-aminobutyric acid (GABA)A receptor-chloride channel ligand t-[35S]butylbicyclophosphorothionate (IC50 of 80 nM) and enhanced binding of the benzodiazepine site ligand [3H]flunitrazepam (EC50 of 125 nM) and the GABA site ligand [3H]muscimol (EC50 of 86 nM), consistent with activity as a positive allosteric modulator of the GABA(A) receptor. Electrophysiological recordings showed that, whereas nanomolar concentrations of Ganaxolone potentiated GABA-evoked chloride currents in Xenopus oocytes expressing the human GABA(A) receptor subunits alpha1beta1gamma2L, alpha2beta1gamma2L or alpha3beta1gamma2L, direct activation of chloride flux occurred to a limited extent only at micromolar concentrations. Ganaxolone was effective in nontoxic doses against clonic convulsions induced by s.c. pentylenetetrazol administration in mice and rats (ED50 values of 4.3 and 7.8 mg/kg i.p., respectively). Ganaxolone also exhibited potent anticonvulsant activity against seizures induced by s.c. bicuculline (ED50 of 4.6 mg/kg i.p.), i.p. TBPS (ED50 of 11.7 mg/kg i.p.) and i.p. aminophylline (ED50 of 11.5 mg/kg i.p.) in mice. Although Ganaxolone effectively blocked tonic seizures induced by maximal electroshock in mice (ED50 of 29.7 mg/kg i.p.), it did so only at doses that produced ataxia on the Rotorod (TD50 of 33.4 mg/kg i.p.). Conversely, Ganaxolone was a potent anticonvulsant against fully kindled stage 5 seizures induced by corneal kindling in rats (ED50 of 4.5 mg/kg i.p.), producing these effects at doses well below those that resulted in ataxia (TD50 of 14.2 mg/kg i.p.). The seizure threshold, as determined by an increase in the dose of i.v. infused pentylenetetrazol required to induce clonus, was also significantly elevated by nontoxic doses of Ganaxolone in mice. In summary, these data indicate that Ganaxolone is a high-affinity, stereoselective, positive allosteric modulator of the GABA(A) receptor complex that exhibits potent anticonvulsant activity across a range of animal procedures. The profile of anticonvulsant activity obtained for Ganaxolone supports clinical evaluation of this drug as an antiepileptic therapy with potential utility in the treatment of generalized absence seizures as well as simple and complex partial seizures.


