GSK744 (S/GSK1265744)HIV integrase inhibitor, oral active and long-acting CAS# 1051375-10-0 |
- S/GSK1349572
Catalog No.:BCC2138
CAS No.:1051375-16-6
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Raltegravir (MK-0518)
Catalog No.:BCC2137
CAS No.:518048-05-0
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
- Elvitegravir (GS-9137)
Catalog No.:BCC2134
CAS No.:697761-98-1
- MK-2048
Catalog No.:BCC2136
CAS No.:869901-69-9
Quality Control & MSDS
Number of papers citing our products

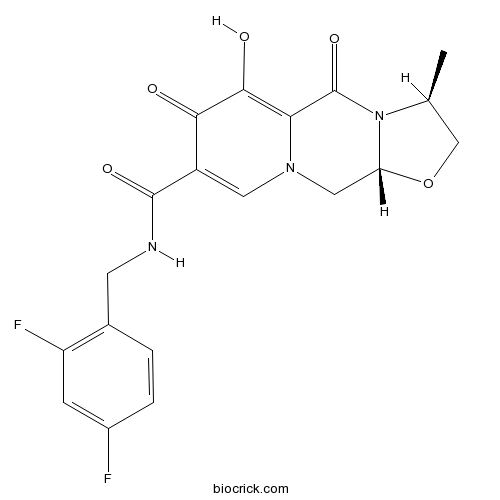
Chemical structure
3D structure
| Cas No. | 1051375-10-0 | SDF | Download SDF |
| PubChem ID | 54713659 | Appearance | Powder |
| Formula | C19H17F2N3O5 | M.Wt | 405.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GSK-1265744; S/GSK1265744 | ||
| Solubility | DMSO : 16.67 mg/mL (41.12 mM; Need ultrasonic) | ||
| SMILES | CC1COC2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)O | ||
| Standard InChIKey | WCWSTNLSLKSJPK-LKFCYVNXSA-N | ||
| Standard InChI | InChI=1S/C19H17F2N3O5/c1-9-8-29-14-7-23-6-12(16(25)17(26)15(23)19(28)24(9)14)18(27)22-5-10-2-3-11(20)4-13(10)21/h2-4,6,9,14,26H,5,7-8H2,1H3,(H,22,27)/t9-,14+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GSK744 is an oral and long-acting inhibitor of HIV integrase. | |||||
| Targets | HIV integrase | |||||

GSK744 (S/GSK1265744) Dilution Calculator

GSK744 (S/GSK1265744) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.467 mL | 12.335 mL | 24.67 mL | 49.3401 mL | 61.6751 mL |
| 5 mM | 0.4934 mL | 2.467 mL | 4.934 mL | 9.868 mL | 12.335 mL |
| 10 mM | 0.2467 mL | 1.2335 mL | 2.467 mL | 4.934 mL | 6.1675 mL |
| 50 mM | 0.0493 mL | 0.2467 mL | 0.4934 mL | 0.9868 mL | 1.2335 mL |
| 100 mM | 0.0247 mL | 0.1234 mL | 0.2467 mL | 0.4934 mL | 0.6168 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK744 (S/GSK1265744) is a potential inhibitor of HIV integrase with IC50 value of 3 nM [1].
HIV integrase is an enzyme produced by HIV virus that enables its genetic material to be integrated into the infected cell DNA. It is reported that HIV intergrase inhibitor plays an important role in halting HIV progression [2].
GSK744 (S/GSK1265744) is a potent HIV integrase inhibitor. Using resistance passage experiments, integrase enzyme assays, and cellular assays with site-directed molecular (SDM) HIV clones resistant to other classes of anti-HIV-1 agents and earlier integrase strand transfer inhibitors, results showed that GSK1265744 efficiently inhibited HIV replication through inhibiting HIV integrase [1].
In female pigtail macaques model that intravaginal inoculated simian/human immunodeficiency virus twice a week for up to 11 weeks, GSK744 injection prevented the macaques from being infected by virus while placebo controls were infected after a 4 median vaginal challenges with SHIV which reminded that GSK744 may be a potential preexposure prophylaxis drug for prevention via inhibiting HIV integrase [3] [2].
Many clinical trials have been conducted to show that GSK744 can efficiently protected healthy subjects from HIV infection [4-6].
References:
[1]. Yoshinaga, T., et al., Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection. Antimicrob Agents Chemother, 2015. 59(1): p. 397-406.
[2]. Andrews, C.D., et al., Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science, 2014. 343(6175): p. 1151-4.
[3]. Radzio, J., et al., The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Transl Med, 2015. 7(270): p. 270ra5.
[4]. Ford, S.L., et al., Lack of pharmacokinetic interaction between rilpivirine and integrase inhibitors dolutegravir and GSK1265744. Antimicrob Agents Chemother, 2013. 57(11): p. 5472-7.
[5]. Spreen, W., et al., Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr, 2014. 67(5): p. 487-92.
[6]. Spreen, W.R., D.A. Margolis, and J.C. Pottage, Jr., Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS, 2013. 8(6): p. 565-71.
- Ligucyperonol
Catalog No.:BCN6638
CAS No.:105108-20-1
- Moellendorffilin
Catalog No.:BCN3546
CAS No.:105099-87-4
- Ro 51
Catalog No.:BCC6157
CAS No.:1050670-85-3
- 1-Ketoaethiopinone
Catalog No.:BCN3219
CAS No.:105062-36-0
- GPR120 modulator 2
Catalog No.:BCC1600
CAS No.:1050506-87-0
- GPR120 modulator 1
Catalog No.:BCC1599
CAS No.:1050506-75-6
- AST-1306 TsOH
Catalog No.:BCC4043
CAS No.:1050500-29-2
- Pallidol
Catalog No.:BCN3306
CAS No.:105037-88-5
- Ascomycin(FK 520)
Catalog No.:BCC1370
CAS No.:104987-12-4
- Tacrolimus (FK506)
Catalog No.:BCC4952
CAS No.:104987-11-3
- 3-Acetoxy-4,7(11)-cadinadien-8-one
Catalog No.:BCN5865
CAS No.:104975-02-2
- PS 1145 dihydrochloride
Catalog No.:BCC7949
CAS No.:1049743-58-9
- S/GSK1349572
Catalog No.:BCC2138
CAS No.:1051375-16-6
- GSK1349572 sodiuM salt
Catalog No.:BCC6407
CAS No.:1051375-19-9
- AC 264613
Catalog No.:BCC3952
CAS No.:1051487-82-1
- 9-Oxoageraphorone
Catalog No.:BCN5866
CAS No.:105181-06-4
- Q94 hydrochloride
Catalog No.:BCC6281
CAS No.:1052076-77-3
- PSB 0739
Catalog No.:BCC6095
CAS No.:1052087-90-7
- PSB 06126
Catalog No.:BCC7417
CAS No.:1052089-16-3
- WEB 2086
Catalog No.:BCC7335
CAS No.:105219-56-5
- Virosine B
Catalog No.:BCN6742
CAS No.:1052228-70-2
- SBE 13 HCl
Catalog No.:BCC6408
CAS No.:1052532-15-6
- 5'-Methoxylariciresinol
Catalog No.:BCN7012
CAS No.:105256-12-0
- 3'-Deoxy-4-O-methylepisappanol
Catalog No.:BCN3676
CAS No.:1052714-12-1
New and investigational antiretroviral drugs for HIV infection: mechanisms of action and early research findings.[Pubmed:23363694]
Top Antivir Med. 2012 Dec;20(5):162-7.
Numerous investigational antiretroviral agents are in clinical development. Among them are festinavir (BMS986001), a thymidine analogue similar to stavudine with reduced potential for toxicity; GS-7340, a prodrug of tenofovir that achieves greater intracellular concentrations; MK-1439, a nonnucleoside analogue reverse transcriptase inhibitor (NNRTI) that retains activity against common NNRTI-associated resistance mutations; and albuvirtide, a long-acting parenteral fusion inhibitor. Investigational integrase strand transfer inhibitors (InSTIs) include elvitegravir, recently approved by the US Food and Drug Administration (FDA) as part of a once-daily, single-tablet formulation with cobicistat/tenofovir/emtricitabine; dolutegravir, which maintains some activity against raltegravir- and elvitegravir-resistant mutants; and S/GSK1265744, which also maintains some activity against resistance mutations in the integrase gene and is being developed as a long-lasting parenteral agent. Novel 2-(quinolin-3-yl)acetic acid derivatives (LEDGINs), agents that were originally thought to inhibit the interaction of integrase with its cofactor lens epithelium-derived growth factor p75 (LEDGF/p75), be active against InSTI-resistant mutants and to have additive activity when combined with InSTIs. This article summarizes a presentation by Michael S. Saag, MD, at the IAS-USA live Improving the Management of HCV Disease continuing medical education program held in New York in October 2012.
Effects of etravirine on the pharmacokinetics of the integrase inhibitor S/GSK1265744.[Pubmed:23114768]
Antimicrob Agents Chemother. 2013 Jan;57(1):277-80.
HIV integrase inhibitors such as raltegravir and elvitegravir halt HIV progression, but treatment-emergent resistance and cross-resistance have been observed. The nonnucleoside reverse transcriptase inhibitor etravirine (ETR) may be used in combination with integrase inhibitors in patients with drug resistance. This single-center, open-label, two-period, single-sequence crossover study evaluated the effects of ETR coadministration on the pharmacokinetic profile of S/GSK1265744, an investigational integrase inhibitor in phase 2 studies. Healthy subjects received 30 mg of S/GSK1265744 alone once daily for 10 days (period 1) and in combination with 200 mg of ETR twice daily for 14 days (period 2). Serial plasma samples for pharmacokinetic analyses were collected on day 10 during period 1 and on day 14 during period 2. All treatments were well tolerated. Etravirine had no effects on S/GSK1265744 geometric mean ratios of the area under the curve from time zero until the end of the dosing interval (1.01; 90% confidence interval [CI], 0.956 to 1.06), of the maximum observed plasma concentration (1.04; 90% CI, 0.987 to 1.09), or of the plasma concentration at the end of the dosing interval (0.999; 90% CI, 0.942 to 1.06). Etravirine pharmacokinetics (PK) parameters observed following coadministration with S/GSK1265744 were in the range of historical values reported for ETR alone in healthy subjects. These results indicate that 30 mg of S/GSK1265744 for 10 days as monotherapy followed by an additional 14 days in combination with ETR was well tolerated in healthy subjects and that no dose adjustment of S/GSK1265744 is required when it is coadministered with ETR.
Parenteral patent drug S/GSK1265744 has the potential to be an effective agent in pre-exposure prophylaxis against HIV infection.[Pubmed:24738551]
Recent Pat Antiinfect Drug Discov. 2013 Dec;8(3):213-8.
The continuing HIV epidemic has driven advancements in antiretroviral therapy. New therapeutic targets have been identified over the past years, one of which has been the Integrase enzyme. This is responsible for integrating HIV pro-DNA into the host cell genome and has proved a successful drug target. Efforts have also been made to improve the pharmacokinetic parameters of current drug therapy and utilise these techniques in maximising drug therapeutic effect whilst minimising adverse events. An exciting example of new technologies is that of nanotechnology where drugs can be specifically targeted to certain tissues and drug delivery can be improved by utilising biological molecules and structures. Pre-exposure prophylaxis is also an area of much interest currently both on an individual and population level. Compliance is however a major issue with daily medication to prevent HIV acquisition as has been demonstrated with contraceptive agents. However if long acting compounds can be developed, compliance can be improved. The patent drug currently being developed through nanotechnology as an analogue of Dolutegravir, GSK1265744 LAP (Long Acting Parenteral) has shown promise as a Long Acting Integrase Inhibitor with potential action both as a therapeutic agent but also in pre-exposure prophylaxis. The favourable pharmacokinetic profile and therapeutic efficacy in comparison to other compounds of the same class demonstrate it to be a promising advance. However given current limitations in study material, further randomised studies with long term follow up are required to fully evaluate the value of the patent drug GSK1265744 LAP in action in both seropositive and seronegative individuals.
Next-generation integrase inhibitors : where to after raltegravir?[Pubmed:23413196]
Drugs. 2013 Mar;73(3):213-28.
The integrase enzyme facilitates the incorporation of HIV-1 proviral DNA into the host cell genome and catalyses a function vital to viral replication. Inhibitors of this enzyme represent the newest class of antiretroviral drugs in our armamentarium to treat HIV-1 infection. Raltegravir, an integrase strand transfer inhibitor, was the first drug of this class approved by the US FDA; it is a potent and well tolerated antiviral agent. However, it has the limitations of twice-daily dosing and a relatively modest genetic barrier to the development of resistance. These qualities have prompted the search for agents with once-daily dosing, a more robust barrier to resistance, and a resistance profile of limited overlap with that of raltegravir. We review a series of integrase inhibitors that are in clinical or advanced pre-clinical studies. Elvitegravir, recently approved by the FDA as part of the elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine fixed-dose combination pill has the benefit of being part of a one-pill, once-daily regimen, but suffers from extensive cross-resistance with raltegravir. Dolutegravir is the most advanced second-generation integrase inhibitor, and it boasts good tolerability, once-daily dosing with no need for a pharmacological enhancer, and relatively little cross-resistance with raltegravir. S/GSK1265744 has been developed into a long-acting parenteral agent that shows a high barrier to resistance in vitro and the potential for an infrequent dosing schedule. BI 224436 is in early clinical trials, but is unlikely to demonstrate cross-resistance with other integrase inhibitors. The inhibitors of the lens epithelium-derived growth factor (LEDGF)/p75 binding site of integrase (LEDGINs) are extremely early in development. Each of these contributes a new benefit to the class and will extend the treatment options for patients with HIV-1 infection.
Carbamoyl pyridone HIV-1 integrase inhibitors 3. A diastereomeric approach to chiral nonracemic tricyclic ring systems and the discovery of dolutegravir (S/GSK1349572) and (S/GSK1265744).[Pubmed:23845180]
J Med Chem. 2013 Jul 25;56(14):5901-16.
We report herein the discovery of the human immunodeficiency virus type-1 (HIV-1) integrase inhibitors dolutegravir (S/GSK1349572) (3) and S/GSK1265744 (4). These drugs stem from a series of carbamoyl pyridone analogues designed using a two-metal chelation model of the integrase catalytic active site. Structure-activity studies evolved a tricyclic series of carbamoyl pyridines that demonstrated properties indicative of once-daily dosing and superior potency against resistant viral strains. An inherent hemiaminal ring fusion stereocenter within the tricyclic carbamoyl pyridone scaffold led to a critical substrate controlled diastereoselective synthetic strategy whereby chiral information from small readily available amino alcohols was employed to control relative and absolute stereochemistry of the final drug candidates. Modest to extremely high levels of stereochemical control were observed depending on ring size and position of the stereocenter. This approach resulted in the discovery of 3 and 4, which are currently in clinical development.


