AST-1306 TsOHErbB2 and EGFR inhibitor CAS# 1050500-29-2 |
- AP26113
Catalog No.:BCC1069
CAS No.:1197958-12-5
- LDK378 dihydrochloride
Catalog No.:BCC1694
CAS No.:1380575-43-8
- ALK inhibitor 1
Catalog No.:BCC1339
CAS No.:761436-81-1
- ALK inhibitor 2
Catalog No.:BCC1340
CAS No.:761438-38-4
- (R)-Crizotinib
Catalog No.:BCC1284
CAS No.:877399-52-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1050500-29-2 | SDF | Download SDF |
| PubChem ID | 25027665 | Appearance | Powder |
| Formula | C31H26ClFN4O5S | M.Wt | 621.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AST-1306 (p-Toluenesulfonic acid); AST1306 (p-Toluenesulfonic acid); AST 1306 (p-Toluenesulfonic acid) | ||
| Solubility | DMSO : 50 mg/mL (80.50 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
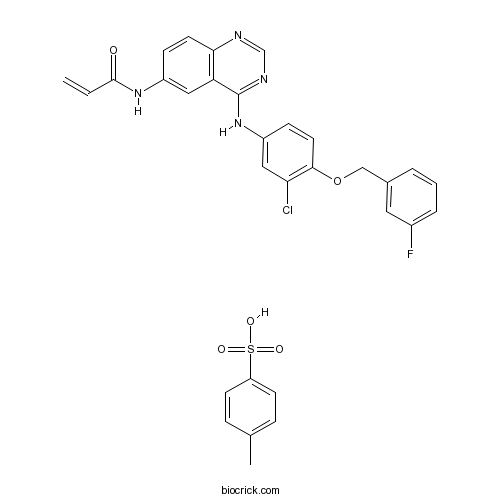
| Chemical Name | N-[4-[3-chloro-4-[(3-fluorophenyl)methoxy]anilino]quinazolin-6-yl]prop-2-enamide;4-methylbenzenesulfonic acid | ||
| SMILES | CC1=CC=C(C=C1)S(=O)(=O)O.C=CC(=O)NC1=CC2=C(C=C1)N=CN=C2NC3=CC(=C(C=C3)OCC4=CC(=CC=C4)F)Cl | ||
| Standard InChIKey | ZMUKJEHWLJBODV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H18ClFN4O2.C7H8O3S/c1-2-23(31)29-17-6-8-21-19(11-17)24(28-14-27-21)30-18-7-9-22(20(25)12-18)32-13-15-4-3-5-16(26)10-15;1-6-2-4-7(5-3-6)11(8,9)10/h2-12,14H,1,13H2,(H,29,31)(H,27,28,30);2-5H,1H3,(H,8,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AST-1306 TsOH is a selective and irreversible inhibitor of ErbB2 and EGFR with IC50 values of 0.5 nM and 3 nM, respectively. | |||||
| Targets | ErbB2 | EGFR | ||||
| IC50 | 0.5 nM | 3 nM | ||||

AST-1306 TsOH Dilution Calculator

AST-1306 TsOH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6101 mL | 8.0505 mL | 16.101 mL | 32.202 mL | 40.2525 mL |
| 5 mM | 0.322 mL | 1.6101 mL | 3.2202 mL | 6.4404 mL | 8.0505 mL |
| 10 mM | 0.161 mL | 0.805 mL | 1.6101 mL | 3.2202 mL | 4.0252 mL |
| 50 mM | 0.0322 mL | 0.161 mL | 0.322 mL | 0.644 mL | 0.805 mL |
| 100 mM | 0.0161 mL | 0.0805 mL | 0.161 mL | 0.322 mL | 0.4025 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AST-1306 is a selective, irreversible inhibitor of ErbB2 and EGFR with IC50 values of 0.5nM and 3nM, respectively [1].
AST-1306 is designed and synthesized based on the chemical structure of lapatinib. The molecular docking method shows AST-1306 binds to the ATP-binding pocket of the kinases and form covalent bind with certain amino acids. AST-1306 can inhibit EGFR and ErbB2 in a cell-free assay with more than 3000-fold selectivity to other kinases. Besides the wild-type EGFR, AST-1306 also inhibits EGFR mutant T790M/L858R both in a cell-free assay and in HIH3T3 cells. The growth of the cells is suppressed by AST-1306 due to the inhibition of the phosphorylation of EGFR. It also occurs in some human cancer cells. Experiments have proved that AST-1306 notly inhibits the phosphorylation of EGFR and ErbB2 and subsequently decreases the downstream pathways of these kinases in A549 cells, Calu-3 cells and SK-OV-3 cells. Moreover, AST-1306 potently inhibits the tumor growth both in ErbB2-overexpressing xenograft models and FVB-2/Nneu transgenic mouse model [1].
References:
[1] Xie H, Lin L, Tong L, Jiang Y, Zheng M, Chen Z, Jiang X, Zhang X, Ren X, Qu W, Yang Y, Wan H, Chen Y, Zuo J, Jiang H, Geng M, Ding J. AST1306, a novel irreversible inhibitor of the epidermal growth factor receptor 1 and 2, exhibits antitumor activity both in vitro and in vivo. PLoS One. 2011;6(7):e21487.
- Pallidol
Catalog No.:BCN3306
CAS No.:105037-88-5
- Ascomycin(FK 520)
Catalog No.:BCC1370
CAS No.:104987-12-4
- Tacrolimus (FK506)
Catalog No.:BCC4952
CAS No.:104987-11-3
- 3-Acetoxy-4,7(11)-cadinadien-8-one
Catalog No.:BCN5865
CAS No.:104975-02-2
- PS 1145 dihydrochloride
Catalog No.:BCC7949
CAS No.:1049743-58-9
- Cardiogenol C hydrochloride
Catalog No.:BCC7790
CAS No.:1049741-55-0
- A 331440 dihydrochloride
Catalog No.:BCC7963
CAS No.:1049740-32-0
- Naspm trihydrochloride
Catalog No.:BCC7476
CAS No.:1049731-36-3
- 8-Hydroxydigitoxigenin
Catalog No.:BCN5864
CAS No.:1049674-06-7
- Ethyl β-D-ribo-hex-3-ulopyranoside
Catalog No.:BCC8977
CAS No.:104953-08-4
- H-D-Val-OtBu.HCl
Catalog No.:BCC3146
CAS No.:104944-18-5
- Tranilast Sodium
Catalog No.:BCC4091
CAS No.:104931-56-8
- GPR120 modulator 1
Catalog No.:BCC1599
CAS No.:1050506-75-6
- GPR120 modulator 2
Catalog No.:BCC1600
CAS No.:1050506-87-0
- 1-Ketoaethiopinone
Catalog No.:BCN3219
CAS No.:105062-36-0
- Ro 51
Catalog No.:BCC6157
CAS No.:1050670-85-3
- Moellendorffilin
Catalog No.:BCN3546
CAS No.:105099-87-4
- Ligucyperonol
Catalog No.:BCN6638
CAS No.:105108-20-1
- GSK744 (S/GSK1265744)
Catalog No.:BCC3888
CAS No.:1051375-10-0
- S/GSK1349572
Catalog No.:BCC2138
CAS No.:1051375-16-6
- GSK1349572 sodiuM salt
Catalog No.:BCC6407
CAS No.:1051375-19-9
- AC 264613
Catalog No.:BCC3952
CAS No.:1051487-82-1
- 9-Oxoageraphorone
Catalog No.:BCN5866
CAS No.:105181-06-4
- Q94 hydrochloride
Catalog No.:BCC6281
CAS No.:1052076-77-3
Synergistic Effect of the TiCl4/p-TsOH Promoter System on the Aza-Prins Cyclization.[Pubmed:26736061]
J Org Chem. 2016 Feb 5;81(3):849-59.
A novel aza-Prins cyclization promoted by a synergistic combination between a Lewis acid and a Bronsted acid to efficiently afford piperidines is described. Contrary to what has been previously reported in the literature, the generality of the reaction employing N-alkyl, N-aryl, and nonprotected homoallylamines has been demonstrated. The reaction is highly diastereoselective depending on the homoallylic amine used, N-PMP homoallyl amine leading preferentially to the trans diastereomer, and free homoallylamine affording the deprotected piperidine as single cis diastereomer.
Chemoselective hydroamination of vinyl arenes catalyzed by an NHC-amidate-alkoxide Pd(II) complex and p-TsOH.[Pubmed:24039309]
Tetrahedron Lett. 2013 Jul 31;54(31):4083-4085.
The hydroamination of various substituted vinyl arenes with benzenesulfonamide was explored using an NHC-amidate-alkoxide palladium catalyst in conjunction with p-TsOH. Utilizing halide-substituted and electron-rich vinyl arenes, this methodology selectively furnished the cross-coupled hydroamination products in moderate to excellent yields in a Markovnikov fashion while greatly reducing undesired acid-catalyzed homocoupling of the vinyl arenes. Electron-rich vinyl arenes typically required milder conditions than electron-poor ones. While most effective for para-substituted substrates, the catalyst system also furnished the desired products from ortho- and meta-substituted vinyl arenes with high chemoselectivities.
p-TsOH Promoted Au(I)-Catalyzed Consecutive Endo Cyclization of Yne-Tethered Ynamide: Access to Benzofused Dihydroisoquinolones.[Pubmed:26524487]
Org Lett. 2015 Nov 20;17(22):5662-5.
A novel synthetic route to benzo[f]dihydroisoquinolone through a p-TsOH promoted cascade cyclization of easily accessible diyne-tethered ynamides in the presence of a Au(I)-catalyst is described. This reaction unveils a broad substrate scope, constructing a wide range of benzo[f]dihydroisoquinolones in good yields. The diyne-tethered ynamides are synthesized from inexpensive o-iodoaniline through Sonogashira couplings and the Cu-mediated C-N bond formation. The role of p-TsOH is examined, and the reaction pathway is also deduced. The benzo[f]isoquinoline scaffold is constructed from benzo[f]dihydroisoquinolones.
Generation of ortho-quinone methides by p-TsOH on silica and their hetero-Diels-Alder reactions with styrenes.[Pubmed:19385608]
J Org Chem. 2009 May 15;74(10):4009-12.
2-Arylchromans were readily prepared from the hetero-Diels-Alder reactions of styrenes with the ortho-quinone methides (o-QMs) which, in turn, were generated by treating the MOM-protected benzylacetate derivatives with p-TsOH immobilized on silica (PTS-Si) in toluene under mild conditions (0 degrees C to rt). The corresponding chromans were obtained in moderate to excellent yields (42-97%) and in moderate to excellent diastereoselectivity (up to >99:1).


