Ergosta-7,22-dien-3-oneCAS# 32507-77-0 |
Quality Control & MSDS
Number of papers citing our products

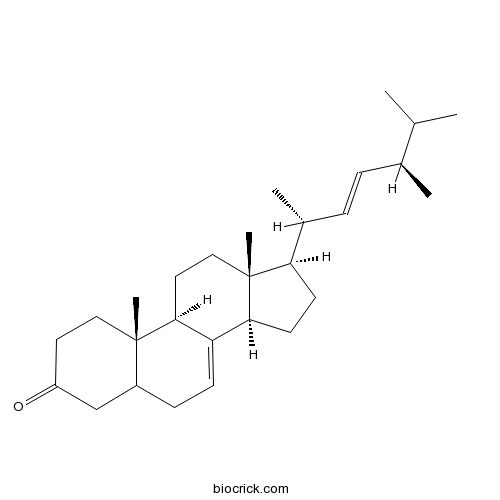
Chemical structure

3D structure
| Cas No. | 32507-77-0 | SDF | Download SDF |
| PubChem ID | 99511 | Appearance | Powder |
| Formula | C28H44O | M.Wt | 396.65 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (9R,10S,13R,14R,17R)-17-[(2R,5R)-5,6-dimethylhept-3-en-2-yl]-10,13-dimethyl-1,2,4,5,6,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one | ||
| SMILES | CC(C)C(C)C=CC(C)C1CCC2C1(CCC3C2=CCC4C3(CCC(=O)C4)C)C | ||
| Standard InChIKey | AHWOEMBXZXGDBQ-AQGSFBQWSA-N | ||
| Standard InChI | InChI=1S/C28H44O/c1-18(2)19(3)7-8-20(4)24-11-12-25-23-10-9-21-17-22(29)13-15-27(21,5)26(23)14-16-28(24,25)6/h7-8,10,18-21,24-26H,9,11-17H2,1-6H3/t19-,20+,21?,24+,25-,26-,27-,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Ergosta-7,22-dien-3-one shows pro-inflammatory property, it can stimulate nitric oxide production, induce the expression of genes, and induce the production of TLRs, cytokines, chemokines, and cellular adhesion molecules in J774A.1 cells. |
| Targets | LTR | NO |

Ergosta-7,22-dien-3-one Dilution Calculator

Ergosta-7,22-dien-3-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5211 mL | 12.6056 mL | 25.2111 mL | 50.4223 mL | 63.0279 mL |
| 5 mM | 0.5042 mL | 2.5211 mL | 5.0422 mL | 10.0845 mL | 12.6056 mL |
| 10 mM | 0.2521 mL | 1.2606 mL | 2.5211 mL | 5.0422 mL | 6.3028 mL |
| 50 mM | 0.0504 mL | 0.2521 mL | 0.5042 mL | 1.0084 mL | 1.2606 mL |
| 100 mM | 0.0252 mL | 0.1261 mL | 0.2521 mL | 0.5042 mL | 0.6303 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isorhapotogenin
Catalog No.:BCN3383
CAS No.:32507-66-7
- Myricanone
Catalog No.:BCN5243
CAS No.:32492-74-3
- Periplocymarin
Catalog No.:BCN8485
CAS No.:32476-67-8
- T 0156 hydrochloride
Catalog No.:BCC5803
CAS No.:324572-93-2
- 1-Indanamine hydrochloride
Catalog No.:BCC8467
CAS No.:32457-23-1
- NPS-2143 hydrochloride
Catalog No.:BCC1808
CAS No.:324523-20-8
- Isochlorogenic acid
Catalog No.:BCN5910
CAS No.:534-61-2
- 20(29)-Lupene-3,23-diol
Catalog No.:BCN5242
CAS No.:32451-85-7
- Khasianine
Catalog No.:BCN2530
CAS No.:32449-98-2
- H-D-Cys-OH.H2O.HCl
Catalog No.:BCC2913
CAS No.:32443-99-5
- Isofebrifugine
Catalog No.:BCN3270
CAS No.:32434-44-9
- Vitexin-4'-Rhamnoside(Rg)
Catalog No.:BCC8369
CAS No.:32426-34-9
- Sulfo-NHS-SS-Biotin
Catalog No.:BCC3580
CAS No.:325143-98-4
- ICA 110381
Catalog No.:BCC6338
CAS No.:325457-99-6
- 1-Oleoyl lysophosphatidic acid sodium salt
Catalog No.:BCC7792
CAS No.:325465-93-8
- Ascleposide E
Catalog No.:BCN5244
CAS No.:325686-49-5
- Indiplon
Catalog No.:BCC7720
CAS No.:325715-02-4
- BPIPP
Catalog No.:BCC7730
CAS No.:325746-94-9
- GRI 977143
Catalog No.:BCC2401
CAS No.:325850-81-5
- Kaempferol 3-neohesperidoside
Catalog No.:BCN5245
CAS No.:32602-81-6
- Oleuropein
Catalog No.:BCN5246
CAS No.:32619-42-4
- Boc-Ser-OH
Catalog No.:BCC3439
CAS No.:3262-72-4
- Fraxamoside
Catalog No.:BCN5247
CAS No.:326594-34-7
- Mesoridazine Besylate
Catalog No.:BCC3975
CAS No.:32672-69-8
Antiinflammatory triterpenoids and steroids from Ganoderma lucidum and G. tsugae.[Pubmed:17655889]
Phytochemistry. 2008 Jan;69(1):234-9.
The antiinflammatory properties of triterpenoids and steroids from both Ganoderma lucidum and Ganoderma tsugae were studied. Twelve compounds, including ergosta-7,22-dien-3beta-ol (1), ergosta-7,22-dien-3beta-yl palmitate (2), Ergosta-7,22-dien-3-one (3), ergosta-7,22-dien-2beta,3alpha,9alpha-triol (4), 5alpha,8alpha-epidioxyergosta-6,22-dien-3beta-ol (5), ganoderal A (6), ganoderal B (7), ganoderic aldehyde A (8), tsugaric acid A (9), 3-oxo-5alpha-lanosta-8,24-dien-21-oic acid (10), 3alpha-acetoxy-5alpha-lanosta-8,24-dien-21-oic acid ester beta-d-glucoside (11), and tsugaric acid B (12), were assessed in vitro by determining their inhibitory effects on the chemical mediators released from mast cells, neutrophils, and macrophages. Compound 10 showed a significant inhibitory effect on the release of beta-glucuronidase from rat neutrophils stimulated with formyl-Met-Leu-Phe (fMLP)/cytochalasin B (CB) whereas compound 9 significantly inhibited superoxide anion formation in fMLP/CB-stimulated rat neutrophils. Compound 10 also exhibited a potent inhibitory effect on NO production in lipopolysaccharide (LPS)/interferon-gamma (IFN-gamma)-stimulated N9 microglial cells. Moreover, compound 9 was also able to protect human keratinocytes against damage induced by ultraviolet B (UV B) light, which indicated 9 could protect keratinocytes from photodamage.
In Vitro Expression of Toll-Like Receptors and Proinflammatory Molecules Induced by Ergosta- 7,22-Dien-3-One Isolated from a Wild Mexican Strain of Ganoderma oerstedii (Agaricomycetes).[Pubmed:28605335]
Int J Med Mushrooms. 2017;19(3):203-211.
Compounds showing pharmacological activity on the immune system are of interest because of their therapeutic potential in the treatment of many diseases. However, data from primary human immune cells and in vivo studies are limited. The aim of this study was to analyze the ability to induce the expression of Toll-like receptors (TLRs) and proinflammatory molecules on cells involved in the immune system using the compound ergosta-7,22-dien-3- one, isolated from a wild Mexican strain of Ganoderma oerstedii. According to our study, Ergosta-7,22-dien-3-one did not present any cytotoxic effect on HeLa or J774A.1 cells, and it was able to stimulate nitric oxide production, induce the expression of genes, and induce the production of TLRs, cytokines, chemokines, and cellular adhesion molecules in J774A.1 cells, based on reverse-transcriptase polymerase chain reaction and enzyme-linked immunosorbent assay. Here we report a new pro-inflammatory property of Ergosta-7,22-dien-3-one, which should be considered as a possible adjuvant property in view of its biological activity.
Isolation and Characterization of Bioactive Metabolites from Fruiting Bodies and Mycelial Culture of Ganoderma oerstedii (Higher Basidiomycetes) from Mexico.[Pubmed:26349508]
Int J Med Mushrooms. 2015;17(6):501-9.
Various species of the genus Ganoderma have been used for centuries according to oriental tradition as a source of medicines and nutrients. A chemical study of the fruiting bodies and mycelial culture of G. oerstedii was carried out with the idea of isolating and characterizing active natural components present to make use of their potential pharmaceutical application in Mexico. The fruiting bodies and mycelial culture of G. oesrtedii were lyophylized and extracted one after the other with hexane, chloroform, and methanol. Following this process, each substance was extracted separately by using column chromatography. From fruiting bodies eight metabolites, five sterols (ergosta-7,22-dien-3beta-ol, ergosterol peroxide, ergosterol, cerevisterol, and Ergosta-7,22-dien-3-one) as well as three terpene compounds (ganodermanondiol, ganoderic acid Sz, and ganoderitriol M) were obtained from fruiting bodies. From the mycelial culture three metabolites, two sterols (ergosterol and cerevisterol), and a new terpene compound (ganoderic acetate from the acid) were obtained. These structures were established based on a spectroscopic analysis mainly using nuclear magnetic resonance and a comparison with data already established.
Cytotoxic constituents of Lasiosphaera fenzlii on different cell lines and the synergistic effects with paclitaxel.[Pubmed:26382563]
Nat Prod Res. 2016 Aug;30(16):1862-5.
The fruit body of Lasiosphaera fenzlii was found to show cytotoxicity on cancer cells during a preliminary screening. Repeated column chromatography of the fungal methanol extract resulted in the isolation of six compounds identified as 5alpha,8alpha-epidioxy-ergosta-6,22-dien-3beta-ol (1), 5alpha,8alpha-epidioxy-ergosta-6,9(11),22-trien-3beta-ol (2), 5alpha-ergosta-7,22-dien-3beta-ol (3), 5alpha-Ergosta-7,22-dien-3-one (4), ergosta-7,22-dien-3beta,5alpha,6beta-triol (5) and 6-dihydroxy-2,3-dihydro-1H-isoindol-1-one (6). The two peroxide compounds, 1 and 2, showed cytotoxic activity and compound 1 was selectively cytotoxic to cancer cells. Furthermore, compound 1 synergised the cytotoxicity of paclitaxel on Hela cells by increasing intracellular accumulation of paclitaxel in cancer cells but not in normal cells.
[Chemical constituents of Osmanthus fragrans fruits].[Pubmed:24791540]
Zhongguo Zhong Yao Za Zhi. 2013 Dec;38(24):4329-34.
By Silica gel, Sephadex LH-20 and other materials for isolation and purification and by physicochemical methods and spectral analysis for structural identification, 23 compounds were isolated and identified from ethyl acetate portion of alcohol extract solution of Osmanthus fragrans fruits. Their structures were identified as nicotinamide (1), D-allitol (2), 5-hydroxymethyl-2-furancarboxaldehyde (3), acetyloleanolic acid (4), benzoic acid (5), Ergosta-7,22-dien-3-one (6), beta-sitosterol (7), borreriagenin (8), cerevistero (9), c-veratroylglycol (10), methyl-2-O-beta-glucopyranosylbenzoate (11), 3', 7-dihydroxy-4'-methoxyisoflavon (12), umbelliferone (13), caffeic acid methyl ester (14), oleanolic acid (15), (-) -chicanine (16), dillapiol (17), 3beta,5alpha, 9alpha-trihydroxyergosta-7-22-dien-6-one (18), 2alpha-hydroxy-oleanolic acid (19), betulinic acid (20), betulin (21), 3, 3'-bisdemethylpinoresinol (22), and lupeol (23). All compounds were isolated from the osmanthus fruit for the first time. Except for compounds 4, 7, 15, 19, 23, the rest ones were isolated from the this plant for the first time.
Antimicrobial effect of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes) and its main compounds.[Pubmed:24940906]
Int J Med Mushrooms. 2014;16(1):77-84.
Mushrooms are considered one of the richest sources of natural antibiotics, and various species of them inhibit the growth of a wide diversity of microorganisms. Ganoderma lucidum, a well-known medicinal mushroom. has many pharmacological and biological activities including an antimicrobial effect, although few studies have investigated the antibacterial and antifungal effects of its purified compounds. The chemical structure of the purified compounds from the hexane fraction was elucidated as ergosta-7,22-dien-3beta-yl acetate, ergosta-5,7,22-trien-3beta-yl acetate (isopyrocalciferol acetate), Ergosta-7,22-dien-3-one, ergosta-7,22-dien-3beta-ol, and ergosta-5,7,22-trien-3beta-ol (ergostrol). In addition, the structure of ganodermadiol was demonstrated after purification from the chloroform fraction. The fractions inhibited Gram-positive bacteria and yeast, with minimum inhibitory concentration values of 6.25 mg/mL, but were ineffective against Gram-negative bacteria in the tested concentrations. The results were comparable for isolated compounds, whereas the mixture of ergosta-7,22-dien-3beta-yl acetate and isopyrocalciferol acetate was weakly effective against Escherichia coli (minimum inhibitory concentration, 10 mg/mL). It could be assumed that the antimicrobial effect of crude fractions is the consequence of mixing triterpenoid and steroid compounds.


