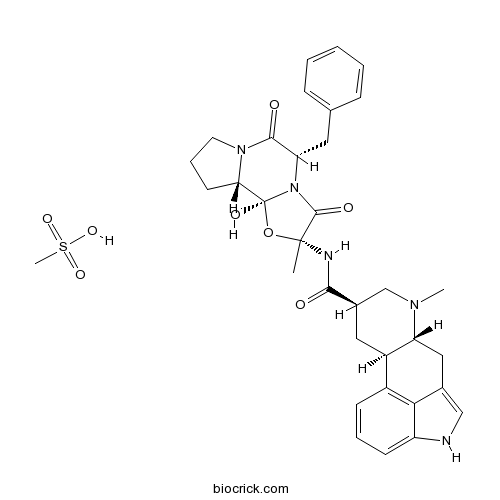
Dihydroergotamine mesylate5-HT antagonist. Also partial agonist at adrenergic and dopaminergic D2 receptors CAS# 6190-39-2 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 6190-39-2 | SDF | Download SDF |
| PubChem ID | 71171 | Appearance | Powder |
| Formula | C34H41N5O8S | M.Wt | 679.78 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (73.55 mM; Need ultrasonic) | ||
| Chemical Name | 9,10-Dihydro-12'-hydroxy-2'-methyl-5'- | ||
| SMILES | CN1C[C@@H](C[C@H]2[C@H]1Cc3c[nH]c4cccc2c34)C(=O)N[C@]5(C)O[C@@]6(O)[C@@H]7CCCN7C(=O)[C@H](Cc8ccccc8)N6C5=O.C[S](O)(=O)=O | ||
| Standard InChIKey | ADYPXRFPBQGGAH-UMYZUSPBSA-N | ||
| Standard InChI | InChI=1S/C33H37N5O5.CH4O3S/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32;1-5(2,3)4/h3-6,8-11,17,21,23,25-27,34,42H,7,12-16,18H2,1-2H3,(H,35,39);1H3,(H,2,3,4)/t21-,23-,25-,26+,27+,32-,33+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Partial α-adrenergic agonist. Partial D2 dopaminergic receptor agonist. Competitive 5-HT antagonist. |

Dihydroergotamine mesylate Dilution Calculator

Dihydroergotamine mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4711 mL | 7.3553 mL | 14.7106 mL | 29.4213 mL | 36.7766 mL |
| 5 mM | 0.2942 mL | 1.4711 mL | 2.9421 mL | 5.8843 mL | 7.3553 mL |
| 10 mM | 0.1471 mL | 0.7355 mL | 1.4711 mL | 2.9421 mL | 3.6777 mL |
| 50 mM | 0.0294 mL | 0.1471 mL | 0.2942 mL | 0.5884 mL | 0.7355 mL |
| 100 mM | 0.0147 mL | 0.0736 mL | 0.1471 mL | 0.2942 mL | 0.3678 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sceleratine
Catalog No.:BCN2126
CAS No.:6190-25-6
- 4-Nitrocinnamic acid
Catalog No.:BCN5033
CAS No.:619-89-6
- p-Ethoxybenzoic acid
Catalog No.:BCN3378
CAS No.:619-86-3
- 4-Hydroxybenzamide
Catalog No.:BCN4147
CAS No.:619-57-8
- 3-(1,1-Dimethylallyl)-8-hydroxy-7-methoxycoumarin
Catalog No.:BCN7570
CAS No.:61899-42-1
- Vernolide B
Catalog No.:BCN6749
CAS No.:618860-58-5
- H-β-homo-Gln-OH.HCl
Catalog No.:BCC2648
CAS No.:61884-74-0
- Demethoxy-7-O-methylcapillarisin
Catalog No.:BCN6469
CAS No.:61854-37-3
- Demethoxycapillarisin
Catalog No.:BCN4611
CAS No.:61854-36-2
- Epoprostenol
Catalog No.:BCC7534
CAS No.:61849-14-7
- Benzyl p-coumarate
Catalog No.:BCN7717
CAS No.:61844-62-0
- Vorapaxar
Catalog No.:BCC3996
CAS No.:618385-01-6
- Tomatidine hydrochloride
Catalog No.:BCN2861
CAS No.:6192-62-7
- Boc-Cys(pMeBzl)-OH
Catalog No.:BCC3377
CAS No.:61925-77-7
- Deoxycalyciphylline B
Catalog No.:BCN4145
CAS No.:619326-74-8
- Deoxyisocalyciphylline B
Catalog No.:BCN4146
CAS No.:619326-75-9
- 9-Hydroxythymol
Catalog No.:BCN7979
CAS No.:61955-76-8
- Norarecoline hydrochloride
Catalog No.:BCN8401
CAS No.:6197-39-3
- Cucurbitacin B
Catalog No.:BCN5919
CAS No.:6199-67-3
- Adrenalone HCl
Catalog No.:BCC4323
CAS No.:62-13-5
- Dopamine hydrochloride
Catalog No.:BCN2195
CAS No.:62-31-7
- Phenacetin
Catalog No.:BCC4436
CAS No.:62-44-2
- Lipoic acid
Catalog No.:BCN5980
CAS No.:62-46-4
- H-Aib-OH
Catalog No.:BCC3207
CAS No.:62-57-7
Where is dihydroergotamine mesylate in the changing landscape of migraine therapy?[Pubmed:21080856]
Expert Opin Pharmacother. 2010 Dec;11(18):3085-93.
IMPORTANCE OF THE FIELD: Migraine affects approximately 18% of women and 6% of men, and has an immense impact on quality of life and productivity. Advancement in therapeutic options has been slow. For many patients with difficult-to-treat migraine, the appropriate use of Dihydroergotamine mesylate (DHE) can result in treatment success and unprecedented patient satisfaction. AREAS COVERED IN THIS REVIEW: Migraine treatment guidelines regarding the role of DHE are highlighted. An overview of the market for antimigraine drugs is provided in the context of DHE, since its introduction in 1943, and the novel agents that are likely to be available in the near future. An extensive literature search was undertaken using Medline and the Cochrane Systematic Review and Clinical Trial databases. WHAT THE READER WILL GAIN: An understanding of which migraine patients are likely to benefit maximally from treatment with DHE in its various forms. TAKE HOME MESSAGE: In the most difficult patient groups - including those with status migrainosus, migraine recurrence, medication-overuse headache, and chronic daily headache - DHE has therapeutic efficacy superior to other agents. The side-effect profile of DHE is more benign than is often perceived and should not be a deterrent for use in well-chosen cases.
Intrapulmonary and intravenous administrations of dihydroergotamine mesylate have similar cardiovascular effects in the conscious dog.[Pubmed:18500365]
Br J Pharmacol. 2008 Jul;154(6):1254-65.
BACKGROUND AND PURPOSE: The effects of intrapulmonary artery (i.p.a.) administration of Dihydroergotamine mesylate (DHE) were evaluated. EXPERIMENTAL APPROACH: Conscious beagle dogs (n=4) were given DHE via the i.p.a. or i.v. route as two 0.014 mg kg(-1) doses and a 0.14 mg kg(-1) dose given 60 min apart. A recovery period of > or =45 h occurred before crossover to the alternative route. Physiological parameters were monitored by telemetry or direct measurement, and venous blood samples were collected for pharmacokinetic assessments. KEY RESULTS: No meaningful differences between i.v. and i.p.a. treatments were observed for heart rate, systemic pressures and vascular pressures. Aortic resistance increased 8, 27 and 70%, respectively, following three doses of i.v. DHE compared with 11, 37 and 57%, respectively, with i.p.a. DHE. Carotid artery resistance increased 22, 40 and 87%, respectively, following three doses of i.v. DHE, compared with 17, 45 and 67%, respectively, following i.p.a. DHE. Increases in coronary artery resistance were of similar magnitude following i.v. and i.p.a. DHE administration. Increases in left ventricular systolic and diastolic pressures were seen following all doses of i.v. and i.p.a. DHE. Changes following DHE 0.014 mg kg(-1) were minimal and not clinically significant. With DHE 0.14 mg kg(-1) by either route, emesis was the most common adverse event. CONCLUSIONS AND IMPLICATIONS: DHE has comparable effects delivered via simulated deep inhalation (i.p.a.) or i.v. administration. The risk of cardiovascular complications is unlikely to be greater following inhalation of DHE.
MAP0004: dihydroergotamine mesylate inhalation aerosol for acute treatment of migraine.[Pubmed:22860628]
Expert Opin Pharmacother. 2012 Sep;13(13):1961-8.
INTRODUCTION: Dihydroergotamine mesylate (DHE) has been used as an acute migraine treatment since 1945, although tolerability with intravenous administration has limited its use. MAP0004 is a novel, orally inhaled, aerosol formulation of DHE that provides pulmonary drug delivery using a pressurized, metered dose inhaler for rapid absorption through lung alveoli. MAP0004 was developed to provide the anti-migraine efficacy of DHE, with fewer systemic effects than intravenous dosing. AREAS COVERED: This review discusses available literature describing the pharmacokinetics, tolerability and efficacy of MAP0004, including data from Phase II and Phase III clinical trials. EXPERT OPINION: MAP0004 aerosol DHE provides desirable activation of 5-HT1B/D receptors, resulting in effective anti-migraine effects. Unlike intravenous DHE, MAP0004 is less likely to bind with other serotonergic, adrenergic and dopaminergic receptors, resulting in fewer unwanted side effects. In addition, MAP0004 is less arterioconstrictive than intravenous DHE. Both Phase II and III clinical trials support anti-migraine efficacy with superior tolerability with MAP0004 compared with intravenous DHE. Inhaled rather than intravenous administration should also improve patient acceptance. These data support the future use of MAP0004 as a first-line acute migraine treatment.
Safety and pharmacokinetics of dihydroergotamine mesylate administered via a Novel (Tempo) inhaler.[Pubmed:18179563]
Headache. 2008 Mar;48(3):355-67.
OBJECTIVE: We investigated the pulmonary absorption of dihydroergotamine (DHE) mesylate and compared the safety, pharmacokinetic, and metabolic profile of 4 different doses of orally inhaled DHE delivered by the Tempo Inhaler (MAP Pharmaceuticals Inc., Mountain View, CA, USA) with 1.0 mg intravenously (IV) administered DHE in 18 healthy subjects. METHODS: Safety was measured by monitoring adverse events, vital signs, electrocardiograms, spirometry, and changes in biochemical and hematological laboratory values. Liquid chromatography, tandem mass spectrometry was used to determine plasma DHE levels while C(max), t(max), AUC(0-6), AUC(0-48), AUC(0-inf), and t(1/2) of parent DHE and the major bioactive metabolite, 8'OH-DHE. Pharmacokinetic parameters and qualitative spectrograms for DHE and metabolites for all treatment groups were compared after inhaled DHE (MAP0004) and IV DHE 1.0 mg. Geometric means and 90% confidence intervals of log-transformed data were calculated and the ratio of means compared. RESULTS: Inhaled DHE resulted in rapid systemic absorption with pharmacokinetic parameters of both parent DHE and 8'OH-DHE similar to those achieved after a 3-minute IV infusion. Post-peak (t(max) approximately 12 minutes) DHE concentrations achieved after 4 actuations ( approximately 0.88 mg respirable dose) of MAP0004 were comparable to those detected after IV administration. The systemic exposure to DHE after 6 actuations of MAP0004 was slightly greater than that achieved after IV administration (geometric mean AUC(0-inf) ratio = 1.24). CONCLUSION: The 4-actuation delivery was well tolerated and provided systemic levels of DHE and 8'OH-DHE slightly lower than IV administration and predicted levels.
Agonistic properties of alniditan, sumatriptan and dihydroergotamine on human 5-HT1B and 5-HT1D receptors expressed in various mammalian cell lines.[Pubmed:9605573]
Br J Pharmacol. 1998 Apr;123(8):1655-65.
1. Alniditan, a novel migraine abortive agent, is a potent 5-HT1B/5-HT1D receptor agonist of nM affinity. We compared the agonistic properties of alniditan, sumatriptan and dihydroergotamine on the cloned human 5-HT1B receptor expressed at 200 fmol mg(-1) protein (Bmax) in non-induced L929sA cells, at 740 fmol mg(-1) protein in HEK 293 and at 2300 fmol mg(-1) protein in mIFNbeta-induced L929sA cells, and on the human cloned 5-HT1D receptor expressed in C6 glioma cells (Bmax 780 fmol mg(-1) protein). 2. Sodium butyrate treatment increased the expression level of human (h)5-HT1B receptors in HEK 293 cells and h5-HT1D receptors in C6 glioma cells approximately 3 fold, the binding affinities of [3H]-5-HT and [3H]-alniditan were unaffected. 3. Agonistic properties were evaluated based on inhibition of cyclic AMP accumulation in the cells after stimulation of adenylyl cyclase by forskolin or isoproterenol. Alniditan, sumatriptan and dihydroergotamine were full agonists at the hS-HT1B receptor (IC50 values were 1.7, 20 and 2 nM, respectively in HEK 293 cells) and hS-HT1D receptors (IC50 values of 1.3, 2.6 and 2.2 nM, respectively). At the h5-HT1B receptor the agonist potency of the compounds slightly increased with higher receptor density. The opposite was seen for antagonists (ocaperidone, risperidone and ritanserin). 4. This comparative study demonstrated that alniditan was 10 times more potent than sumatriptan at the h5-HT1B receptor, and twice as potent at the h5-HT1D receptor. Dihydroergotamine was more potent an agonist at the h5-HT1B receptor when expressed at high and low level in L929sA cells (but not in HEK 293 cells), and was less potent at the hS-HT1D receptor.
Further analysis of the inhibitory effects of dihydroergotamine, cyproheptadine and ketanserin on the responses of the rat aorta to 5-hydroxytryptamine.[Pubmed:1512277]
J Auton Pharmacol. 1992 Aug;12(4):223-36.
1. The aim of the present study was to analyse the inhibitory effects of dihydroergotamine, cyproheptadine and ketanserin on the rat aorta contractile responses to 5-HT. Initially phenoxybenzamine treatment was used to determine whether spare receptors exist at the maximum responses to 5-HT. Then the reversibility of the inhibitory effects of phentolamine, dihydroergotamine, cyproheptadine and ketanserin against the fast and slow response of the rat aorta to 5-HT were determined. 2. Phenoxybenzamine caused non-parallel rightward shifts of the 5-HT concentration-response curves with reduced maximal responses. The KA values for 5-HT to produce fast and slow responses were 2-5 x 10(-5) M. These KA values are much higher than those previously reported for 5-HT at 5-HT2-receptors and suggest that the 5-HT2-receptor of the rat aorta is 'atypical'. The rat aorta has spare 5-HT-receptors for the maximal fast and slow responses to 5-HT. 3. Phentolamine, dihydroergotamine, cyproheptadine and ketanserin inhibited the fast and slow responses to 5-HT. Phentolamine and ketanserin and the higher concentrations of dihydroergotamine and cyproheptadine tested had greater inhibitory effects on the fast than slow 5-HT responses possibly because the fast 5-HT response did not always reach equilibrium in the presence of antagonists. 4. The inhibitory effects on 5-HT responses of phentolamine at 10(-6)-10(-5) M were readily reversible, those of cyproheptadine at 10(-9)-10(-8) M and ketanserin at 10(-8) M were slowly reversible and those of dihydroergotamine at 10(-9)-10(-8) M were irreversible by washing in drug-free Krebs. 5. The inhibitory effects of phentolamine and ketanserin were not altered by increasing the treatment time whereas some of the effects of cyproheptadine and the effects of dihydroergotamine on responses to 5-HT were increased by prolonging the contact time with the antagonist from 75 to 150 min. 6. The present study shows that at the rat aorta 5-HT2-receptor, phentolamine is a competitive readily reversible, cyproheptadine and ketanserin are competitive slowly reversible and dihydroergotamine is a competitive irreversible antagonist.
Effects of S9977 and dihydroergotamine in an animal experimental model for migraine.[Pubmed:1635891]
Pharmacol Res. 1992 Feb-Mar;25(2):125-37.
The present study concerns the effects of S9977, a trimethylxanthine derivative with potential antimigraine characteristics, on the distribution of carotid blood flow in the anaesthetized pig. Furthermore, the effects of dihydroergotamine have been analysed for comparison. Dihydroergotamine (3, 10, 30 and 100 micrograms/kg, i.v.) elicited dose-dependent pressor and bradycardic responses which were probably mediated by its partial agonist action on alpha-adrenoceptors and dopamine2 receptors. In contrast, S9977 (0.3, 1, 3 and 10 mg/kg, i.v.) caused a moderate hypotension and bradycardia. The carotid haemodynamic effects of dihydroergotamine (3, 10, 30 and 100 micrograms/kg, i.v.) consisted of a dose-dependent reduction of arteriovenous anastomotic blood flow and conductance and an increase in nutrient (tissue) blood flow and conductance. As a consequence, jugular venous PO2 decreased. These findings, demonstrating an active constriction of arteriovenous anastomoses, are in agreement with earlier findings in cats. Though S9977 (0.3, 1, 3 and 10 mg/kg, i.v.) decreased carotid (two highest doses) and arteriovenous anastomotic (highest dose) blood flow, there was no concomitant decrease in the vascular conductances. Therefore, the effects of S9977 seem to be related to a decrease in arterial blood pressure and not to an active vasoconstriction of arteriovenous anastomoses. These results are discussed in terms of the potential therapeutical usefulness of S9977 in the treatment of migraine.
Carotid vascular effects of ergotamine and dihydroergotamine in the pig: no exclusive mediation via 5-HT1-like receptors.[Pubmed:1664762]
Br J Pharmacol. 1991 Sep;104(1):183-9.
1. Though it is well known that the antimigraine drugs ergotamine and dihydroergotamine reduce carotid arteriovenous anastomotic shunting, it is uncertain whether a 5-HT1-like receptor is responsible for this effect. Using a high dose of methiothepin (3 mg kg-1), which completely blocks the carotid vascular effects of sumatriptan, we have attempted to study the role of 5-HT1-like receptors in the carotid vascular effects of ergotamine as well as dihydroergotamine in anaesthetized pigs. 2. Both ergotamine and dihydroergotamine increased arterial blood pressure and decreased heart rate. 3. The ergot alkaloids reduced dose-dependently total carotid blood flow and conductance as a result of a selective decrease in the arteriovenous anastomotic fraction. The nutrient fraction increased, particularly to bones, tongue and salivary glands with ergotamine and to ears, head skin, bones and salivary glands with dihydroergotamine. In contrast, dural vascular conductance tended to decrease. 4. Methiothepin (3 mg kg-1) partially antagonized the decrease in total carotid and arteriovenous anastomotic blood flow and conductance by the ergot alkaloids; the ED30 for ergotamine and dihydroergotamine (agonist dose eliciting a 30% decrease in arteriovenous anastomotic conductance) was raised by 3.1 and 5.2 fold respectively. 5. These results indicate that the effects of ergotamine and dihydroergotamine are partly mediated by methiothepin-sensitive receptors, which may probably belong to either 5-HT1-like or alpha 2-adrenoceptor category. However, an important part of the effect of ergot alkaloids is left after methiothepin and this could be mediated by other, perhaps novel, receptors.


