4-HydroxybenzamideCAS# 619-57-8 |
Quality Control & MSDS
Number of papers citing our products

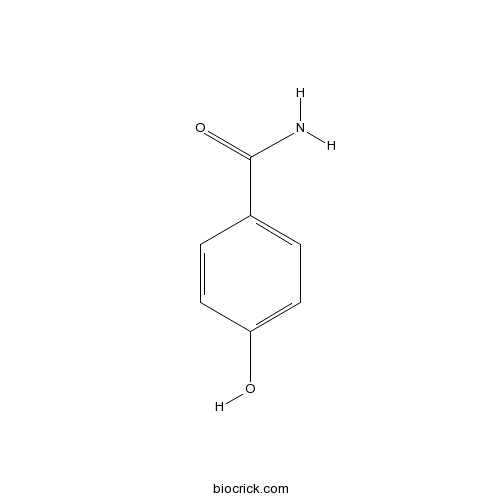
Chemical structure

3D structure
| Cas No. | 619-57-8 | SDF | Download SDF |
| PubChem ID | 65052 | Appearance | Powder |
| Formula | C7H7NO2 | M.Wt | 137.1 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-hydroxybenzamide | ||
| SMILES | C1=CC(=CC=C1C(=O)N)O | ||
| Standard InChIKey | QXSAKPUBHTZHKW-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4-Hydroxybenzamide is a natural product from Berberis pruinosa. |
| Structure Identification | J Phys Chem B. 2015 Aug 20;119(33):10466-77.Influence of Secondary Interactions on the Structure, Sublimation Thermodynamics, and Solubility of Salicylate:4-Hydroxybenzamide Cocrystals. Combined Experimental and Theoretical Study.[Pubmed: 26258951]Cocrystal screening of 4-Hydroxybenzamide with a number of salicylates (salicylic acid, SA; 4-aminosalicylic acid, PASA; acetylsalicylic acid, ASA; and salicylsalicylic acid, SSA) was conducted to confirm the formation of two cocrystals, [SA+4-OHBZA] (1:1) and [PASA+4-OHBZA] (1:1). |

4-Hydroxybenzamide Dilution Calculator

4-Hydroxybenzamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.2939 mL | 36.4697 mL | 72.9395 mL | 145.8789 mL | 182.3487 mL |
| 5 mM | 1.4588 mL | 7.2939 mL | 14.5879 mL | 29.1758 mL | 36.4697 mL |
| 10 mM | 0.7294 mL | 3.647 mL | 7.2939 mL | 14.5879 mL | 18.2349 mL |
| 50 mM | 0.1459 mL | 0.7294 mL | 1.4588 mL | 2.9176 mL | 3.647 mL |
| 100 mM | 0.0729 mL | 0.3647 mL | 0.7294 mL | 1.4588 mL | 1.8235 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-(1,1-Dimethylallyl)-8-hydroxy-7-methoxycoumarin
Catalog No.:BCN7570
CAS No.:61899-42-1
- Vernolide B
Catalog No.:BCN6749
CAS No.:618860-58-5
- H-β-homo-Gln-OH.HCl
Catalog No.:BCC2648
CAS No.:61884-74-0
- Demethoxy-7-O-methylcapillarisin
Catalog No.:BCN6469
CAS No.:61854-37-3
- Demethoxycapillarisin
Catalog No.:BCN4611
CAS No.:61854-36-2
- Epoprostenol
Catalog No.:BCC7534
CAS No.:61849-14-7
- Benzyl p-coumarate
Catalog No.:BCN7717
CAS No.:61844-62-0
- Vorapaxar
Catalog No.:BCC3996
CAS No.:618385-01-6
- Sipeimine
Catalog No.:BCN1201
CAS No.:61825-98-7
- Oxaliplatin
Catalog No.:BCC3932
CAS No.:61825-94-3
- Trans-Melilotoside
Catalog No.:BCC8364
CAS No.:618-67-7
- Ethyl 3,4,5-trimethoxybenzoate
Catalog No.:BCN3973
CAS No.:6178-44-5
- p-Ethoxybenzoic acid
Catalog No.:BCN3378
CAS No.:619-86-3
- 4-Nitrocinnamic acid
Catalog No.:BCN5033
CAS No.:619-89-6
- Sceleratine
Catalog No.:BCN2126
CAS No.:6190-25-6
- Dihydroergotamine mesylate
Catalog No.:BCC5224
CAS No.:6190-39-2
- Tomatidine hydrochloride
Catalog No.:BCN2861
CAS No.:6192-62-7
- Boc-Cys(pMeBzl)-OH
Catalog No.:BCC3377
CAS No.:61925-77-7
- Deoxycalyciphylline B
Catalog No.:BCN4145
CAS No.:619326-74-8
- Deoxyisocalyciphylline B
Catalog No.:BCN4146
CAS No.:619326-75-9
- 9-Hydroxythymol
Catalog No.:BCN7979
CAS No.:61955-76-8
- Norarecoline hydrochloride
Catalog No.:BCN8401
CAS No.:6197-39-3
- Cucurbitacin B
Catalog No.:BCN5919
CAS No.:6199-67-3
- Adrenalone HCl
Catalog No.:BCC4323
CAS No.:62-13-5
Influence of Secondary Interactions on the Structure, Sublimation Thermodynamics, and Solubility of Salicylate:4-Hydroxybenzamide Cocrystals. Combined Experimental and Theoretical Study.[Pubmed:26258951]
J Phys Chem B. 2015 Aug 20;119(33):10466-77.
Cocrystal screening of 4-Hydroxybenzamide with a number of salicylates (salicylic acid, SA; 4-aminosalicylic acid, PASA; acetylsalicylic acid, ASA; and salicylsalicylic acid, SSA) was conducted to confirm the formation of two cocrystals, [SA+4-OHBZA] (1:1) and [PASA+4-OHBZA] (1:1). Their structures were determined using single-crystal X-ray diffraction, and the hydrogen-bond network topology was studied. Thermodynamic characteristics of salicylic acid cocrystal sublimation were obtained experimentally. It was proved that PASA cocrystallization with 4-OHBZA makes the drug more stable and prevents the irreversible process of decarboxylation of PASA resulting in formation of toxic 3-aminophenol. The pattern of non-covalent interactions in the cocrystals is described quantitatively using solid-state density functional theory followed by Bader analysis of the periodic electron density. It has been found that the total energy of secondary interactions between synthon atoms and the side hydroxyl group of the acid molecule in [SA+4-OHBZA] (1:1) and [PASA+4-OHBZA] (1:1) cocrystals is comparable to the energy of the primary acid-amide heterosynthon. The theoretical value of the sublimation enthalpy of [SA+4-OHBZA], 231 kJ/mol, agrees fairly well with the experimental one, 272 kJ/mol. The dissolution experiments with [SA+4-OHBZA] have proved that the relatively large cocrystal stability in relation to the stability of its components has a negative effect on the dissolution rate and equilibrium solubility. The [PASA+4-OHBZA] (1:1) cocrystal showed an enhancement of apparent solubility compared to that of the corresponding pure active pharmaceutical ingredient, while their intrinsic dissolution rates are comparable.


