CEP-18770Proteasome inhibitor CAS# 847499-27-8 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Epoxomicin
Catalog No.:BCC1235
CAS No.:134381-21-8
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
Quality Control & MSDS
Number of papers citing our products

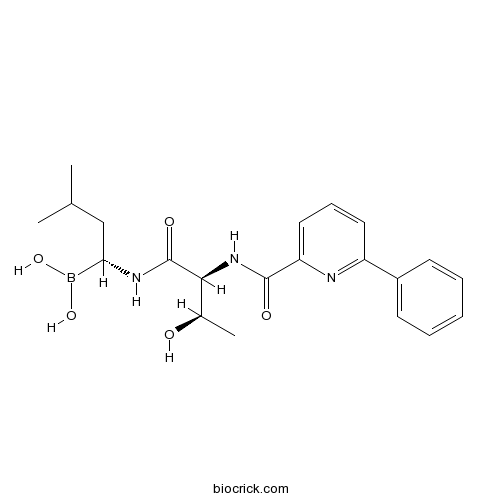
Chemical structure
3D structure
| Cas No. | 847499-27-8 | SDF | Download SDF |
| PubChem ID | 24800541 | Appearance | Powder |
| Formula | C21H28BN3O5 | M.Wt | 413.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CEP-18770 | ||
| Solubility | DMSO : 130 mg/mL (314.56 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | [(1R)-1-[[(2S,3R)-3-hydroxy-2-[(6-phenylpyridine-2-carbonyl)amino]butanoyl]amino]-3-methylbutyl]boronic acid | ||
| SMILES | B(C(CC(C)C)NC(=O)C(C(C)O)NC(=O)C1=CC=CC(=N1)C2=CC=CC=C2)(O)O | ||
| Standard InChIKey | SJFBTAPEPRWNKH-CCKFTAQKSA-N | ||
| Standard InChI | InChI=1S/C21H28BN3O5/c1-13(2)12-18(22(29)30)24-21(28)19(14(3)26)25-20(27)17-11-7-10-16(23-17)15-8-5-4-6-9-15/h4-11,13-14,18-19,26,29-30H,12H2,1-3H3,(H,24,28)(H,25,27)/t14-,18+,19+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CEP-18770 is a novel orally-active inhibitor of the chymotrypsin-like activity of the proteasome with a cellular IC50 value of 3.8 nM. | |||||
| Targets | proteasome | |||||
| IC50 | 3.8 nM | |||||
| Cell experiment: [1] | |
| Cell lines | RPMI-8226 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 40 nM, 4 to 8 hours |
| Applications | As an inhibitor of proteasome, CEP-18770 induced an accumulation of ubiquitinated proteins over 4 to 8 hours with a profile similar to that observed after bortezomib (another proteasome inhibitor) treatment. |
| Animal experiment : [1] | |
| Animal models | SCID mice bearing human MM RPMI 8226 subcutaneous xenograft |
| Dosage form | Intravenous (from 1 to 6 mg/kg, 2q7d×8 injections) or oral administration (in a solution of 3% DMSO, 10% Solutol, and 87% sterile NaCl 0.9%, twice-a-week injections for 4 weeks at doses of 7.8, 10, 13 mg/kg in a volume of 20 mL/kg body weight of mouse). |
| Application | Intravenous administration of CEP-18770 exhibited sustained and dose-related tumor weight inhibition with RTWI of 100% at all tested doses. CEP-18770 also exhibited dose-related increases in the incidence of tumor-free mice by the completion of the studies (120 days after tumor transplantation) within the 3- and 4-mg/kg intravenous treatment groups, 89% and 80%, respectively. Oral administration of CEP-18770 resulted in a significant reduction of tumor weight and notable dose-related incidence of complete tumor regression (75% incidence of CR and 25% tumor-free mice at 10 mg/kg orally). |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Piva R, Ruggeri B, Williams M, et al. CEP-18770: A novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood, 2008, 111(5): 2765-2775. | |

CEP-18770 Dilution Calculator

CEP-18770 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4195 mL | 12.0977 mL | 24.1955 mL | 48.391 mL | 60.4887 mL |
| 5 mM | 0.4839 mL | 2.4195 mL | 4.8391 mL | 9.6782 mL | 12.0977 mL |
| 10 mM | 0.242 mL | 1.2098 mL | 2.4195 mL | 4.8391 mL | 6.0489 mL |
| 50 mM | 0.0484 mL | 0.242 mL | 0.4839 mL | 0.9678 mL | 1.2098 mL |
| 100 mM | 0.0242 mL | 0.121 mL | 0.242 mL | 0.4839 mL | 0.6049 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CEP-18770 is a novel, potent and reversible P2 threonine boronic acid inhibitor of proteasome that inhibits proteasome’s chymotrypsin-like activity, with the value of inhibition constant IC50 of 3.8 nM, by down-modulating the activity of nuclear factor-KB (NF-KB) as well as the expression of a few NF-KB downstream effectors. Preliminary results of multiple studies have shown that CEP-18770 exerts potent antitumor activities against human multiple myeloma (MM) cell lines by inducing apoptotic cell death, exhibits a strong antiangiogenic activity suppressing RANKL-induced osteoclastogenesis, and displays a favorable cytotoxicity profile towards normal cells including epithelial cells, bone marrow progenitors, and bone marrow-derived stromal cells.
Reference
Piva R, Ruggeri B, Williams M, Costa G, Tamagno I, Ferrero D, Giai V, Coscia M, Peola S, Massaia M, Pezzoni G, Allievi C, Pescalli N, Cassin M, di Giovine S, Nicoli P, de Feudis P, Strepponi I, Roato I, Ferracini R, Bussolati B, Camussi G, Jones-Bolin S, Hunter K, Zhao H, Neri A, Palumbo A, Berkers C, Ovaa H, Bernareggi A, Inghirami G. CEP-18770: A novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood. 2008;111(5):2765-2775
- Eriocalyxin B
Catalog No.:BCN4390
CAS No.:84745-95-9
- Qingyangshengenin
Catalog No.:BCN4389
CAS No.:84745-94-8
- Calceolarioside A
Catalog No.:BCN5347
CAS No.:84744-28-5
- UVI 3003
Catalog No.:BCC7638
CAS No.:847239-17-2
- Arglabin
Catalog No.:BCC5299
CAS No.:84692-91-1
- Astragaloside IV
Catalog No.:BCN5960
CAS No.:84687-43-4
- Astragaloside III
Catalog No.:BCN5963
CAS No.:84687-42-3
- Astragaloside I
Catalog No.:BCN5961
CAS No.:84680-75-1
- 3-O-(2'E,4'Z-Decadienoyl)ingenol
Catalog No.:BCN3767
CAS No.:84680-59-1
- Enalaprilat Dihydrate
Catalog No.:BCC5009
CAS No.:84680-54-6
- Astragaloside II
Catalog No.:BCN5962
CAS No.:84676-89-1
- Isoastragaloside I
Catalog No.:BCN2979
CAS No.:84676-88-0
- SHA 68
Catalog No.:BCC6210
CAS No.:847553-89-3
- NVP-BEP800
Catalog No.:BCC2129
CAS No.:847559-80-2
- ICG 001
Catalog No.:BCC3632
CAS No.:847591-62-2
- Tasumatrol L
Catalog No.:BCN6955
CAS No.:847835-17-0
- S 32212 hydrochloride
Catalog No.:BCC6208
CAS No.:847871-78-7
- Lenalidomide hemihydrate
Catalog No.:BCC4198
CAS No.:847871-99-2
- RO4929097
Catalog No.:BCC2089
CAS No.:847925-91-1
- Lck Inhibitor
Catalog No.:BCC1689
CAS No.:847950-09-8
- 5-Epilithospermoside
Catalog No.:BCN4392
CAS No.:84799-31-5
- threo-1-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCN1331
CAS No.:848031-94-7
- 3,4-Dimethoxyphenyl beta-D-glucoside
Catalog No.:BCN4393
CAS No.:84812-00-0
- HKI 357
Catalog No.:BCC6046
CAS No.:848133-17-5
Novel, orally active, proteasome inhibitor, delanzomib (CEP-18770), ameliorates disease symptoms and glomerulonephritis in two preclinical mouse models of SLE.[Pubmed:22178195]
Int Immunopharmacol. 2012 Jan;12(1):257-70.
Current therapies for late-stage systemic lupus erythematosus (SLE) are limited to cytotoxic agents. Delanzomib (CEP-18770) is an orally active, reversible P2 threonine boronic acid inhibitor of the 26S mammalian proteasome. Delanzomib was tested in a head-to-head comparison against bortezomib to protect and treat mice with fatal lupus nephritis (LN). Age matched MRL/lpr or NZBWF1 mice with established SLE or LN, respectively, were treated with delanzomib either 3 mg/kg once or twice weekly intravenously or orally at 10 mg/kg. Mice were also treated with reference agent bortezomib at 0.5 mg/kg, intraperitoneally, once a week or 0.3 mg/kg once or twice a week. Reductions in the frequencies of specific anti-chromatin, smith and dsDNA antibody secreting cells and levels of the corresponding circulating antinuclear antibodies, were observed following delanzomib treatment. Reductions in several serum pro-inflammatory cytokines were observed in delanzomib-treated animals. Delanzomib treatment suppressed the development and progression of renal tissue damage and extended the survival of ill mice. Proteinuria was significantly decreased and severity of various renal histopathologies reduced relative to vehicle-treated nephritic mice. Treatment of lupus in these models demonstrated that delanzomib treatment lead to greater tolerability and rate of response resulting in improved stabilization of disease.
Phase I/II study of the novel proteasome inhibitor delanzomib (CEP-18770) for relapsed and refractory multiple myeloma.[Pubmed:28140719]
Leuk Lymphoma. 2017 Aug;58(8):1872-1879.
Delanzomib (CEP-18770), a reversible P2 threonine boronic acid proteasome (beta5/beta1 subunits) inhibitor that showed promising anti-myeloma effects in preclinical studies, was investigated in a single-agent multicenter phase I/II study in patients with relapsed/refractory myeloma. Sixty-one patients (17 during dose escalation; 44 in the expansion cohort) received delanzomib on days 1, 8, and 15 in 28-d cycles; 47 received the maximum tolerated dose (MTD), 2.1 mg/m(2). Dose-limiting toxicities (DLTs) at 2.4 mg/m(2) were rash and thrombocytopenia. At the MTD, the most prominent adverse events were nausea, vomiting, anorexia, fatigue, and pyrexia; grade 3/4 thrombocytopenia and neutropenia occurred in 53 and 23% of patients, respectively. Peripheral neuropathy (21%) was limited to grades 1/2. At the MTD, 26 patients (55%) had stable disease and four (9%) had a partial response (PR). Median time to progression (TTP) was 2.5 months across the cohort. Based upon the efficacy results, development of delanzomib for myeloma was discontinued.
A first in human phase I study of the proteasome inhibitor CEP-18770 in patients with advanced solid tumours and multiple myeloma.[Pubmed:23058787]
Eur J Cancer. 2013 Jan;49(2):290-6.
BACKGROUND: The safety, pharmacokinetics (PK) and pharmacodynamics of CEP-18770, a new peptide boronic acid proteasome inhibitor, have been investigated after intravenous administration on days 1, 4, 8 and 11 of every 21d cycle in patients with solid tumours and multiple myeloma (MM). PATIENTS AND METHODS: Thirty-eight patients were treated with CEP-18770 at escalating doses from 0.1 to 1.8mg/m(2) where 2 out of 5 patients showed dose limiting toxicities. The maximum tolerated/recommended dose (MTD/RD) of 1.5mg/m(2) was tested in 12 additional patients. Skin rash was dose-limiting and occurred in 53% of patients; other frequent toxicities were asthenia (29%), stomatitis (21%) and pyrexia (16%). No significant peripheral neuropathy was observed. PK in plasma was linear with a half-life of the elimination phase of 62.0+/-43.5h. Proteasome inhibition in peripheral blood mononuclear cells was dose related in MM patients; it was of 45.4+/-11.5% at the RD. CONCLUSIONS: CEP-18770 showed a favourable safety profile with lack of neurotoxicity and linear plasma PK. The definition of the optimal biological dose and schedule of treatment is actively pursued because of the high incidence of skin toxicity of the twice a week schedule.
CEP-18770 (delanzomib) in combination with dexamethasone and lenalidomide inhibits the growth of multiple myeloma.[Pubmed:22906694]
Leuk Res. 2012 Nov;36(11):1422-7.
Preclinical and clinical studies have shown that proteasome inhibitors (PIs) have anti-MM activity in combination with dexamethasone or lenalidomide. However, no data exists on the anti-MM effects of combinations involving the PI delanzomib with dexamethasone and/or lenalidomide. Herein, we show that delanzomib in combination with dexamethasone and/or lenalidomide results in superior tumor reduction and extended tumor growth delays when compared to vehicle alone, these drugs alone, or the doublet of dexamethasone and lenalidomide. The favorable results obtained from the three xenograft studies suggest that delanzomib in combination with dexamethasone and lenalidomide should be explored for the treatment of MM.


