D-MannitolCAS# 69-65-8 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Monomethyl auristatin E
Catalog No.:BCC1775
CAS No.:474645-27-7
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 69-65-8 | SDF | Download SDF |
| PubChem ID | 453 | Appearance | Powder |
| Formula | C6H14O6 | M.Wt | 182.17 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | Mannitol; Mannite;87-78-5;50-70-4;Sorbitol | ||
| Solubility | H2O : ≥ 36 mg/mL (197.62 mM) *"≥" means soluble, but saturation unknown. | ||
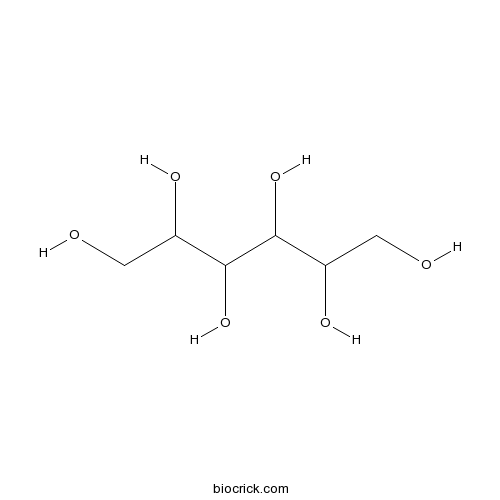
| Chemical Name | hexane-1,2,3,4,5,6-hexol | ||
| SMILES | C(C(C(C(C(CO)O)O)O)O)O | ||
| Standard InChIKey | FBPFZTCFMRRESA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | D-Mannitol, a specific hydroxyl free radical scavenger, can reduce the developmental toxicity of hydroxyurea in rabbits; it has thermal properties and could be as a Phase Change Material (PCM) for latent heat storage system; it is a potential diagnostic marker for aspergillosis. D-mannitol has neuroprotectant effect in reducing the sensory neurological disturbances seen in ciguatera poisoning, it does not act purely as an osmotic agent to reduce swelling of nerves, but involves a more complex action dependent on the Na(v) channel subtype, possibly to alter or reduce toxin association. |
| Targets | Sodium Channel | DNA/RNA Synthesis |
| In vitro | Neuroprotectant effects of iso-osmolar D-mannitol to prevent Pacific ciguatoxin-1 induced alterations in neuronal excitability: a comparison with other osmotic agents and free radical scavengers.[Pubmed: 15950247]Neuropharmacology. 2005 Oct;49(5):669-86.
|
| In vivo | Increased amounts of the Aspergillus metabolite D-mannitol in tissue and serum of rats with experimental aspergillosis.[Pubmed: 2499640]J Infect Dis. 1989 Jul;160(1):95-103.Several Aspergillus species produce large amounts of the hexitol D-Mannitol in vitro, but it is not known whether these species also produce D-Mannitol in vivo.
|
| Animal Research | D-mannitol, a specific hydroxyl free radical scavenger, reduces the developmental toxicity of hydroxyurea in rabbits.[Pubmed: 8073363 ]Teratology. 1994 Apr;49(4):248-59.Hydroxyurea (HU) is a potent mammalian teratogen. Within 2-4 hours after maternal injection, HU causes 1) a rapid episode of embryonic cell death and 2) profound inhibition of embryonic DNA synthesis. A variety of antioxidants delays the onset of embryonic cell death and reduces the incidence of birth defects. Antioxidants do not block the inhibition of DNA synthesis, indicating that early embryonic cell death is not caused by inhibited DNA synthesis. We have suggested that some HU molecules may react within the embryo to produce H2O2 and subsequent free radicals, including the very reactive hydroxyl free radical. The free radicals could cause the early cell death; antioxidants are believed to terminate the aberrant free radical reactions resulting in lessened developmental toxicity.
|
| Structure Identification | J. Appl. Sci., 2011, 11(16):3044-8.Thermal Analysis of D-mannitol for Use as Phase Change Material for Latent Heat Storage.[Reference: WebLink]The aim of this study was to investigate the thermal properties of D-Mannitol as a Phase Change Material (PCM) for latent heat storage system. Heat absorbed by D-Mannitol causes it to undergo a change from the solid to the liquid phase and this heat is stored as the latent heat of fusion. The stored energy can then be retrieved at a later time for various applications.
|

D-Mannitol Dilution Calculator

D-Mannitol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4894 mL | 27.4469 mL | 54.8938 mL | 109.7876 mL | 137.2345 mL |
| 5 mM | 1.0979 mL | 5.4894 mL | 10.9788 mL | 21.9575 mL | 27.4469 mL |
| 10 mM | 0.5489 mL | 2.7447 mL | 5.4894 mL | 10.9788 mL | 13.7234 mL |
| 50 mM | 0.1098 mL | 0.5489 mL | 1.0979 mL | 2.1958 mL | 2.7447 mL |
| 100 mM | 0.0549 mL | 0.2745 mL | 0.5489 mL | 1.0979 mL | 1.3723 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
D-Mannitol
- Penicillin G Sodium
Catalog No.:BCC4638
CAS No.:69-57-8
- Ampicillin
Catalog No.:BCC1199
CAS No.:69-52-3
- Chlorpromazine HCl
Catalog No.:BCC4460
CAS No.:69-09-0
- Quinacrine 2HCl
Catalog No.:BCC4709
CAS No.:69-05-6
- Uncarine A
Catalog No.:BCN7767
CAS No.:6899-73-6
- Mulberrofuran A
Catalog No.:BCN3677
CAS No.:68978-04-1
- SKF 91488 dihydrochloride
Catalog No.:BCC6675
CAS No.:68941-21-9
- Kahweol
Catalog No.:BCC9006
CAS No.:6894-43-5
- H-D-Glu-OH
Catalog No.:BCC2936
CAS No.:6893-26-1
- Guaiacol salicylate
Catalog No.:BCC8327
CAS No.:87-16-1
- Eburicol
Catalog No.:BCN4252
CAS No.:6890-88-6
- Otonecine
Catalog No.:BCN2009
CAS No.:6887-34-9
- Salicylic acid
Catalog No.:BCC4109
CAS No.:69-72-7
- Cytarabine hydrochloride
Catalog No.:BCC4116
CAS No.:69-74-9
- Maltose
Catalog No.:BCC8338
CAS No.:69-79-4
- 2,6-Dihydroxypurine
Catalog No.:BCN8476
CAS No.:69-89-6
- Hypaconitine
Catalog No.:BCN5988
CAS No.:6900-87-4
- Lycodoline
Catalog No.:BCN2506
CAS No.:6900-92-1
- Norchelerythrine
Catalog No.:BCN3643
CAS No.:6900-99-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
- Toltrazuril sulfone
Catalog No.:BCC2008
CAS No.:69004-04-2
- Tiotidine
Catalog No.:BCC5676
CAS No.:69014-14-8
- Genipin
Catalog No.:BCN5932
CAS No.:6902-77-8
- Germacrone
Catalog No.:BCN4981
CAS No.:6902-91-6
D-mannitol, a specific hydroxyl free radical scavenger, reduces the developmental toxicity of hydroxyurea in rabbits.[Pubmed:8073363]
Teratology. 1994 Apr;49(4):248-59.
Hydroxyurea (HU) is a potent mammalian teratogen. Within 2-4 hours after maternal injection, HU causes 1) a rapid episode of embryonic cell death and 2) profound inhibition of embryonic DNA synthesis. A variety of antioxidants delays the onset of embryonic cell death and reduces the incidence of birth defects. Antioxidants do not block the inhibition of DNA synthesis, indicating that early embryonic cell death is not caused by inhibited DNA synthesis. We have suggested that some HU molecules may react within the embryo to produce H2O2 and subsequent free radicals, including the very reactive hydroxyl free radical. The free radicals could cause the early cell death; antioxidants are believed to terminate the aberrant free radical reactions resulting in lessened developmental toxicity. To investigate whether hydroxyl free radicals cause the early episode of cell death, pregnant New Zealand white rabbits were injected subcutaneously on gestational day 12 with a teratogenic dose of HU (650 mg/kg) in the presence or absence of 550 mg/kg of D-Mannitol (Man), a specific scavenger of hydroxyl free radicals. Osmotic control rabbits received HU plus 550 mg/kg of xylose (Xyl, a nonactive aldose). At term, the teratologic effects of HU were ameliorated by Man as evidenced by decreased incidences of the expected limb malformations. Xyl exerted no demonstrable effect on HU teratogenesis. Histological examination of limb buds at 3-8 hours after maternal injection, showed that Man delayed the onset of HU-induced cell death by as much as 4 hours. Xyl had no effect. That Man acts within the embryo was shown by performing intracoelomic injections on alternate implantation sites with Man, Xyl, or saline followed by subcutaneous injection of the pregnant doe with HU. Embryos were harvested 3-8 hours later. Limb buds from saline- and Xyl-injected embryos exhibited the typical pattern of widespread HU-induced cell death at 3-4 hours, whereas Man-injected embryos did not exhibit cell death until 5-8 hours. These results are consistent with those reported for antioxidant-mediated amelioration of HU-induced developmental toxicity and with the hypothesis that hydroxyl free radicals are the proximate reactive species in HU-induced early embryonic cell death.
Inhaled mannitol for non-cystic fibrosis bronchiectasis: a randomised, controlled trial.[Pubmed:25246664]
Thorax. 2014 Dec;69(12):1073-9.
RATIONALE: Bronchiectasis is characterised by excessive production of mucus and pulmonary exacerbations. Inhaled osmotic agents may enhance mucociliary clearance, but few long-term clinical trials have been conducted. OBJECTIVES: To determine the impact of inhaled mannitol on exacerbation rates in patients with non-cystic fibrosis (CF) bronchiectasis. Secondary endpoints included time to first exacerbation, duration of exacerbations, antibiotic use for exacerbations and quality of life (QOL) (St George's Respiratory Questionnaire, SGRQ). METHODS: Patients with non-CF bronchiectasis and a history of chronic excess production of sputum and >/=2 pulmonary exacerbations in the previous 12 months were randomised (1:1) to 52 weeks treatment with inhaled mannitol 400 mg or low-dose mannitol control twice a day. Patients were 18-85 years of age, baseline FEV1 >/=40% and /=30. MAIN RESULTS: 461 patients (233 in the mannitol and 228 in the control arm) were treated. Baseline demographics were similar in the two arms. The exacerbation rate was not significantly reduced on mannitol (rate ratio 0.92, p=0.31). However, time to first exacerbation was increased on mannitol (HR 0.78, p=0.022). SGRQ score was improved on mannitol compared with low-dose mannitol control (-2.4 units, p=0.046). Adverse events were similar between groups. CONCLUSIONS: Mannitol 400 mg inhaled twice daily for 12 months in patients with clinically significant bronchiectasis did not significantly reduce exacerbation rates. There were statistically significant improvements in time to first exacerbation and QOL. Mannitol therapy was safe and well tolerated. TRIAL REGISTRATION NUMBER: NCT00669331.
Neuroprotectant effects of iso-osmolar D-mannitol to prevent Pacific ciguatoxin-1 induced alterations in neuronal excitability: a comparison with other osmotic agents and free radical scavengers.[Pubmed:15950247]
Neuropharmacology. 2005 Oct;49(5):669-86.
The basis for the neuroprotectant effect of D-Mannitol in reducing the sensory neurological disturbances seen in ciguatera poisoning, is unclear. Pacific ciguatoxin-1 (P-CTX-1), at a concentration 10 nM, caused a statistically significant swelling of rat sensory dorsal root ganglia (DRG) neurons that was reversed by hyperosmolar 50 mM D-Mannitol. However, using electron paramagnetic resonance (EPR) spectroscopy, it was found that P-CTX-1 failed to generate hydroxyl free radicals at concentrations of toxin that caused profound effects on neuronal excitability. Whole-cell patch-clamp recordings from DRG neurons revealed that both hyper- and iso-osmolar 50 mM D-Mannitol prevented the membrane depolarisation and repetitive firing of action potentials induced by P-CTX-1. In addition, both hyper- and iso-osmolar 50 mM D-Mannitol prevented the hyperpolarising shift in steady-state inactivation and the rise in leakage current through tetrodotoxin (TTX)-sensitive Na(v) channels, as well as the increased rate of recovery from inactivation of TTX-resistant Na(v) channels induced by P-CTX-1. D-Mannitol also reduced, but did not prevent, the inhibition of peak TTX-sensitive and TTX-resistant I(Na) amplitude by P-CTX-1. Additional experiments using hyper- and iso-osmolar D-sorbitol, hyperosmolar sucrose and the free radical scavenging agents Trolox and L-ascorbic acid showed that these agents, unlike D-Mannitol, failed to prevent the effects of P-CTX-1 on spike electrogenesis and Na(v) channel gating. These selective actions of D-Mannitol indicate that it does not act purely as an osmotic agent to reduce swelling of nerves, but involves a more complex action dependent on the Na(v) channel subtype, possibly to alter or reduce toxin association.


