TiotidinePotent, selective H2 antagonist CAS# 69014-14-8 |
- Dexmedetomidine HCl
Catalog No.:BCC4347
CAS No.:145108-58-3
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Isoprenaline HCl
Catalog No.:BCC4328
CAS No.:51-30-9
- Metoprolol Tartrate
Catalog No.:BCC4330
CAS No.:56392-17-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 69014-14-8 | SDF | Download SDF |
| PubChem ID | 50287 | Appearance | Powder |
| Formula | C10H16N8S2 | M.Wt | 312.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ICI 125,211 | ||
| Solubility | Soluble to 10 mM in ethanol and to 100 mM in DMSO | ||
| Chemical Name | 1-cyano-3-[2-[[2-(diaminomethylideneamino)-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-2-methylguanidine | ||
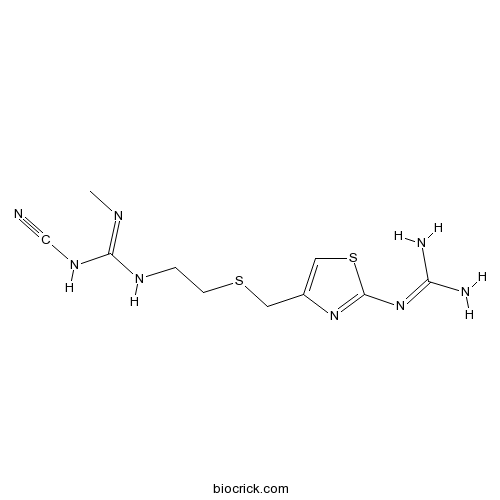
| SMILES | CN=C(NCCSCC1=CSC(=N1)N=C(N)N)NC#N | ||
| Standard InChIKey | YDDXVAXDYKBWDX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H16N8S2/c1-14-9(16-6-11)15-2-3-19-4-7-5-20-10(17-7)18-8(12)13/h5H,2-4H2,1H3,(H2,14,15,16)(H4,12,13,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent histamine H2-receptor antagonist with negligible activity against H1- and H3- receptors. |

Tiotidine Dilution Calculator

Tiotidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2009 mL | 16.0046 mL | 32.0092 mL | 64.0184 mL | 80.023 mL |
| 5 mM | 0.6402 mL | 3.2009 mL | 6.4018 mL | 12.8037 mL | 16.0046 mL |
| 10 mM | 0.3201 mL | 1.6005 mL | 3.2009 mL | 6.4018 mL | 8.0023 mL |
| 50 mM | 0.064 mL | 0.3201 mL | 0.6402 mL | 1.2804 mL | 1.6005 mL |
| 100 mM | 0.032 mL | 0.16 mL | 0.3201 mL | 0.6402 mL | 0.8002 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Toltrazuril sulfone
Catalog No.:BCC2008
CAS No.:69004-04-2
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
- Norchelerythrine
Catalog No.:BCN3643
CAS No.:6900-99-8
- Lycodoline
Catalog No.:BCN2506
CAS No.:6900-92-1
- Hypaconitine
Catalog No.:BCN5988
CAS No.:6900-87-4
- 2,6-Dihydroxypurine
Catalog No.:BCN8476
CAS No.:69-89-6
- Maltose
Catalog No.:BCC8338
CAS No.:69-79-4
- Cytarabine hydrochloride
Catalog No.:BCC4116
CAS No.:69-74-9
- Salicylic acid
Catalog No.:BCC4109
CAS No.:69-72-7
- D-Mannitol
Catalog No.:BCN2205
CAS No.:69-65-8
- Penicillin G Sodium
Catalog No.:BCC4638
CAS No.:69-57-8
- Ampicillin
Catalog No.:BCC1199
CAS No.:69-52-3
- Genipin
Catalog No.:BCN5932
CAS No.:6902-77-8
- Germacrone
Catalog No.:BCN4981
CAS No.:6902-91-6
- ZM 306416
Catalog No.:BCC3964
CAS No.:690206-97-4
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
- Grifolin
Catalog No.:BCN7553
CAS No.:6903-07-7
- Hydroxytyrosol acetate
Catalog No.:BCN2963
CAS No.:69039-02-7
- Nedocromil
Catalog No.:BCC5283
CAS No.:69049-73-6
- Delphinidin-3-O-glucoside chloride
Catalog No.:BCN3020
CAS No.:6906-38-3
- Peonidin-3-O-glucoside chloride
Catalog No.:BCN3028
CAS No.:6906-39-4
- 4'-Prenyloxyresveratrol
Catalog No.:BCN2937
CAS No.:69065-16-3
- 2-Caren-10-ol
Catalog No.:BCN4253
CAS No.:6909-19-9
- Demanyl phosphate
Catalog No.:BCN1796
CAS No.:6909-62-2
Histamine H(2) -like receptors in chick cerebral cortex: effects on cyclic AMP synthesis and characterization by [(3) H]tiotidine binding.[Pubmed:12065605]
J Neurochem. 2002 Jun;81(5):935-46.
In this study, histamine (HA) receptors in chick cerebral cortex were characterized using two approaches: (1) analysis of the effects of HA-ergic drugs on the cAMP-generating system, and (2) radioreceptor binding of [(3) H]Tiotidine, a selective H(2) antagonist. HA was a weak activator of adenylyl cyclase in a crude membrane preparation of chick cerebrum. On the other hand, HA (0.1-1000 microm) potently and concentration dependently stimulated cAMP production in [(3) H]adenine pre-labelled slices of chick cerebral cortex, displaying an EC(50) value (concentration that produces 50% of maximum response) of 2.65 microm. The effect of HA was mimicked by agonists of HA receptors with the following rank order of potency: HA >or= 4-methylHA (H(2)) >or= N alpha,N alpha-dimethylHA (H(3) >> H(2) = H(1)) >> 2-methylHA (H(1)) >> 2-thiazolylethylamine (H(1)) >or= R alpha-methylHA (H(3)) >> amthamine, dimaprit (H(2)), immepip (H(3), H(4)). The HA-evoked increase in cAMP production in chick cerebral cortex was antagonized by selective H(2) receptor blockers (aminopotentidine >or= Tiotidine > ranitidine >> zolantidine), and not significantly affected by mepyramine and thioperamide, selective H(1) and H(3) /H(4) receptor blockers, respectively. A detailed analysis of the antagonistic action of aminopotentidine (vs. HA) revealed a non-competitive mode of action. The binding of [(3) H]Tiotidine to chick cortical membranes was rapid, stable and reversible. Saturation analysis resulted in a linear Scatchard plot, suggesting binding to a single class of receptor binding site with high affinity [equilibrium dissociation constant (K (d)) = 4.42 nm] and high capacity [maximum number of binding sites (B (max) ) = 362 fmol/mg protein]. The relative rank order of HA-ergic drugs to inhibit [(3) H]Tiotidine binding to chick cerebrum was: antagonists - Tiotidine >> aminopotentidine = ranitidine >or= zolantadine >> thioperamide - triprolidine; agonists - HA >or= 4-methylHA >> 2-methylHA >or=R alpha-methylHA - dimaprit. In conclusion, chick cerebral cortex contains H(2) -like HA receptors that are linked to the cAMP-generating system and are labelled with [(3) H]Tiotidine. The pharmacological profile of these receptors is different from that described for their mammalian counterpart. It is suggested that the studied receptors represent either an avian-specific H(2) -like HA receptors or a novel subtype of HA receptors.
Tiotidine, a histamine H2 receptor inverse agonist that binds with high affinity to an inactive G-protein-coupled form of the receptor. Experimental support for the cubic ternary complex model.[Pubmed:12869657]
Mol Pharmacol. 2003 Aug;64(2):512-20.
Knowing the importance for research and pharmacological uses of proper ligand classification into agonists, inverse agonists, and antagonists, the aim of this work was to study the behavior of Tiotidine, a controversial histamine H2 receptor ligand. We found that Tiotidine, described previously as an H2 antagonist, actually behaves as an inverse agonist in U-937 cells, diminishing basal cAMP levels. [3H]Tiotidine showed two binding sites, one with high affinity and low capacity and the other with low affinity and high capacity. The former site disappeared in the presence of guanosine 5'-O-(3-thio)triphosphate, indicating that it belongs to a subset of receptors coupled to G-protein, showing the classic binding profile for an agonist. Considering the occupancy models developed up to now, the only one that explains Tiotidine dual behavior is the cubic ternary complex (CTC) model. This model allows G-protein to interact with the receptor even in the inactive state. We showed by theoretical simulations based on the CTC model of dose-response and binding experiments that Tiotidine biases the system to a G-protein-coupled form of the receptor that is unable to evoke a response. This theoretical approach was supported by experimental results in which an unrelated G-protein-coupled receptor that also signals through Galphas-protein (beta2-adrenoreceptor) was impeded by Tiotidine. This interference clearly implies that Tiotidine biases the system to Galphas-coupled form of the H2 receptor and turns Galphas-protein less available to interact with beta2-adrenoreceptor. These findings not only show that Tiotidine is an H2 inverse agonist in U-937 cells but also provide experimental support for the CTC model.
Characterization of histamine H(2)-like receptors in duck cerebral cortical membranes by [(3)H]tiotidine binding.[Pubmed:11834315]
Neurosci Lett. 2002 Feb 22;319(3):149-52.
A selective (according to mammalian criteria) histamine (HA) H(2)-receptor radioligand [(3)H]Tiotidine ([(3)H]TIOT) was used to characterize HA receptors in duck cerebral cortex by an in vitro binding technique. The specific binding of [(3)H]TIOT to duck cerebral cortical membranes was found to be rapid, stable, saturable, reversible, and of high affinity. Saturation analysis resulted in a linear Scatchard plot suggesting binding to a single class of receptor binding sites with high affinity (K(d)=19.5 nM) and high capacity (B(max)=356 fmol/mg protein). Competition studies showed the following relative rank order of potency of various HA receptor ligands to inhibit the [(3)H]TIOT binding: antagonists, Tiotidine>>ranitidine=zolantidine>or=cimetidine>>mepyramine>thioperamide; agonists, HA>or=4-methylHA>2-methylHA>dimaprit>>R(alpha)-methylHA. The biphasic nature of the displacement curve for HA and the effect of 5'-guanylimidodiphosphate indicate the coupling of the studied receptor to G-protein. It is suggested that HA receptors in the duck cerebral cortex labelled with [(3)H]TIOT represent either avian-specific H(2)-like HA receptors or a novel subtype of HA receptors, coupled to a signalling pathway other than the adenylyl cyclase/cyclic adenosine monophosphate one.
Combined histamine H1/H2 receptor antagonists: part II. Pharmacological hybrids with pheniramine- and tiotidine-like substructures.[Pubmed:9795053]
Eur J Pharm Sci. 1998 Jul;6(3):187-96.
Hybrid molecules combining the crucial structural features of both pheniramine-type histamine H1 receptor antagonists and guanidinothiazole-type H2 receptor antagonists have been synthesized and tested for in vitro pharmacological activity at the isolated ileum and the spontaneously beating right atrium of the guinea-pig. In the title compounds the basic side chain nitrogen of the H1 antagonist and the so-called 'polar group' (cyanoguanidine, urea, or nitroethenediamine) of the H2 antagonist moiety have been linked by a polymethylene spacer. The new substances displayed high affinities to both histamine receptor subtypes and a dual type of antagonism (surmountable/insurmountable) characterized by a shift of the concentration response curves to the right accompanied by a depression of the maximal response to the agonist if the antagonist concentration was >/=100 nM. Highest combined histamine antagonist activities were found in the nitroethenediamine series with pKB values ranging from 8.16 to 9.04 in the ileum (H1) and 7.0-8.08 in the atrium (H2)
Distribution, properties, and functional characteristics of three classes of histamine receptor.[Pubmed:2164693]
Pharmacol Rev. 1990 Mar;42(1):45-83.
It is clear from the preceding overview of histamine receptor pharmacology that research into the pharmacology of histamine receptors is at an exciting stage of development. The rapid advance of molecular biology should soon see the structural identification and cloning of all three of the major vertebrate histamine receptors. Further work will continue toward enhancing our understanding of the control by histamine of intracellular signaling via H1- and H2-receptors, and the rapid explosion of work on the H3-receptor should begin to unravel the mechanisms underlying its actions, perhaps via effects on ionic channels. The potential role of histamine as an intracellular second messenger raises exciting possibilities, as does the search for a histamine receptor analogous to the ligand-gated ion channel in the invertebrate nervous system.
The cardiac pharmacology of tiotidine (LCL 125, 211): a new histamine H2-receptor antagonist.[Pubmed:6105207]
J Pharmacol Exp Ther. 1980 Sep;214(3):629-34.
The cardiac pharmacology of Tiotidine, a potent and specific antagonist of H2-receptors, was studied in isolated Langendorff-perfused guinea-pig hearts. At a concentration of 2.5 x 10(-6) M, Tiotidine caused a slight prolongation of the P-R interval, but had no effect on rate, contractility or coronary function. Tiotidine competitively antagonized the positive chronotropic action of histamine with an apparent dissociation constant of 3.0 x 10(-8) M (2.3 x 10(-8)-3.8 x 10(-8) M). Tiotidine abolished H2-mediated increases in contractile force leaving H2-mediated negative inotropic responses unopposed. The actions of histamine at the A-V node, manifested by lengthening of the P-R interval and A-V block, were attenuated by 2.5 x 10(-7) M Tiotidine; nevertheless, higher concentrations of the antagonist did not produce additional inhibition of the negative dromotropic response. As a function of concentration, Tiotidine reduced the incidence and duration of histamine-induced idioventricular arrhythmias. Based on its high affinity and specificity for the H2-receptor and relative lack of cardio-depressant effects, Tiotidine appears to be a useful new drug for investigating the role of histamine in cardiac function and dysfunction.


