Cilastatin sodiumDipeptidase inhibitor CAS# 81129-83-1 |
- Adefovir Dipivoxil
Catalog No.:BCC5025
CAS No.:142340-99-6
- Valganciclovir HCl
Catalog No.:BCC4745
CAS No.:175865-59-5
- CMX001
Catalog No.:BCC4106
CAS No.:444805-28-1
- Valganciclovir
Catalog No.:BCC2026
CAS No.:88110-89-8
- Letermovir
Catalog No.:BCC1700
CAS No.:917389-32-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 81129-83-1 | SDF | Download SDF |
| PubChem ID | 23663403 | Appearance | Powder |
| Formula | C16H25N2NaO5S | M.Wt | 380.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MK 0791 | ||
| Solubility | Soluble to 100 mM in water and to 10 mM in DMSO | ||
| Chemical Name | (2Z)-7-[[(2R)-2-Amino-2-carboxyethy | ||
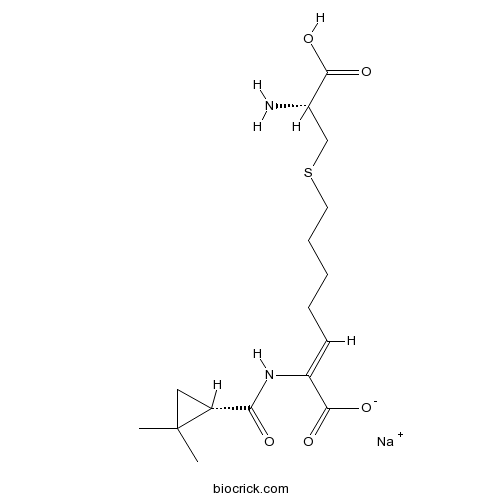
| SMILES | [Na+].CC1(C)C[C@@H]1C(=O)NC(=CCCCCSC[C@@H](N)C(O)=O)/C([O-])=O | ||
| Standard InChIKey | QXPBTTUOVWMPJN-VQAYNFQASA-M | ||
| Standard InChI | InChI=1S/C16H26N2O5S.Na/c1-16(2)8-10(16)13(19)18-12(15(22)23)6-4-3-5-7-24-9-11(17)14(20)21;/h6,10-11H,3-5,7-9,17H2,1-2H3,(H,18,19)(H,20,21)(H,22,23);/q;+1/p-1/b12-6-;/t10-,11-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dipeptidase inhibitor (LTDase, leukotriene D4 hydrolase, dehydropeptidase I) that displays a Ki value of 0.11 μM. Inhibits metabolism of LTD4 to LTE4 and the hydrolysis of β-lactam antibiotics. Nephroprotective; reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells. |

Cilastatin sodium Dilution Calculator

Cilastatin sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6286 mL | 13.143 mL | 26.286 mL | 52.5721 mL | 65.7151 mL |
| 5 mM | 0.5257 mL | 2.6286 mL | 5.2572 mL | 10.5144 mL | 13.143 mL |
| 10 mM | 0.2629 mL | 1.3143 mL | 2.6286 mL | 5.2572 mL | 6.5715 mL |
| 50 mM | 0.0526 mL | 0.2629 mL | 0.5257 mL | 1.0514 mL | 1.3143 mL |
| 100 mM | 0.0263 mL | 0.1314 mL | 0.2629 mL | 0.5257 mL | 0.6572 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (Z)-Lachnophyllum lactone
Catalog No.:BCN4746
CAS No.:81122-95-4
- N-Nonyldeoxynojirimycin
Catalog No.:BCC7752
CAS No.:81117-35-3
- Racecadotril
Catalog No.:BCC4614
CAS No.:81110-73-8
- Clarithromycin
Catalog No.:BCC9219
CAS No.:81103-11-9
- Cisapride
Catalog No.:BCC4207
CAS No.:81098-60-4
- Pravastatin
Catalog No.:BCC4141
CAS No.:81093-37-0
- Kauniolide
Catalog No.:BCC5313
CAS No.:81066-45-7
- EUK 134
Catalog No.:BCC4317
CAS No.:81065-76-1
- Methyl 4-prenyloxycinnamate
Catalog No.:BCN7520
CAS No.:81053-49-8
- 4-Hydroxy-4-(methoxycarbonylmethyl)cyclohexanone
Catalog No.:BCN1346
CAS No.:81053-14-7
- Rhodamine B
Catalog No.:BCN7215
CAS No.:81-88-9
- Warfarin
Catalog No.:BCC5221
CAS No.:81-81-2
- Pravastatin sodium
Catalog No.:BCC2321
CAS No.:81131-70-6
- RR-src
Catalog No.:BCC6956
CAS No.:81156-93-6
- Imiloxan hydrochloride
Catalog No.:BCC6875
CAS No.:81167-22-8
- Apatinib
Catalog No.:BCC5099
CAS No.:811803-05-1
- Boc-D-Tyr(2-Br-Z)-OH
Catalog No.:BCC3464
CAS No.:81189-61-9
- Panaxynol
Catalog No.:BCN3833
CAS No.:81203-57-8
- L-741,626
Catalog No.:BCC6886
CAS No.:81226-60-0
- 15-Deoxoeucosterol
Catalog No.:BCN4348
CAS No.:81241-53-4
- ent-9-Hydroxy-15-oxo-16-kauren-19-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN1345
CAS No.:81263-96-9
- ent-6,11-Dihydroxy-15-oxo-16-kauren-19-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN1344
CAS No.:81263-97-0
- ent-6,9-Dihydroxy-15-oxo-16-kauren-19-oic acid beta-D-glucopyranosyl ester
Catalog No.:BCN1343
CAS No.:81263-98-1
- ent-6,9-Dihydroxy-15-oxo-16-kauren-19-oic acid
Catalog No.:BCN1342
CAS No.:81264-00-8
Imipenem/cilastatin sodium (IPM/CS) as an embolic agent for transcatheter arterial embolisation: a preliminary clinical study of gastrointestinal bleeding from neoplasms.[Pubmed:23961409]
Springerplus. 2013 Jul 26;2:344.
PURPOSE: To evaluate the feasibility and usefulness of imipenem/Cilastatin sodium (IPM/CS) as an embolic agent for intestinal bleeding from neoplasms. MATERIALS AND METHODS: Seven patients who underwent 11 transarterial embolisations (TAEs) using IPM/CS as an embolic material for duodenal or small/large intestinal tumour bleeding from January 2004 to December 2011 were retrospectively evaluated. A mixture of IPM/CS and contrast medium was introduced through the microcatheter positioned at the feeding artery to the tumour until extravasation disappeared or stasis of blood flow to the tumour staining was observed. RESULTS: Haemostasis was obtained in all patients. Therefore, the technical success rate was 100%. Rebleeding was observed in four patients. All of them underwent repeat TAE using IPM/CS, and haemostasis was obtained successfully. No complication was identified following laboratory and clinical examinations. No haemorrhagic death occurred. Haemorrhagic parameters, including blood haemoglobin and the amount of blood transfusion, improved after TAE. CONCLUSION: The safety, feasibility, and effectiveness of TAE using IPM/CS as an embolic material for intestinal bleeding from neoplasms were suggested by this study. The mild embolic effect of IPM/CS may be adequate for oozing from tumours. Although rebleeding may occur after embolotherapy using IPM/CS, repeat embolisation is effective as treatment for rebleeding.
[Domestic imipenem cilastatin sodium for the treatment of severe aspiration pneumonia, a curative effect observation].[Pubmed:23040783]
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2012 Oct;24(10):628-31.
OBJECTIVE: To evaluate the efficacy and safety of domestic imipenem Cilastatin sodium for the treatments of severe aspiration pneumonia. METHODS: A randomize, open, parallel-controlled trial was conducted. Sixty-eight patients with severe aspiration pneumonia were divided into trial group (n=36) and control group (n=32) by random distribution method. The application of trial group domestic imipenem Cilastatin sodium was 1.0 g intravenous drip, every 6-8 hours for 7-14 days. The control group application with imported injection imipenem Cilastatin sodium was 1.0 g intravenous drip, every 6-8 hours for 7-14 days. The highest daily temperature (T), heart rate (HR), breathing rate (RR), pulse blood oxygen saturation (SpO(2)), blood oxygen partial pressure (PaO(2)), inhaled oxygen concentration (FiO(2)), oxygenation index (PaO(2)/FiO(2)), airway peak pressure (Paw), minute ventilation (MV) and white blood count (WBC), pro calcitonin (PCT), high-sensitivity C-reactive protein (hs-CRP) index before and 1, 3, 7 days after treatment, and liver and kidney function, chest X-rays, and sputum cultures of drug sensitive test were conducted. And the effectiveness and safety were determined according to the standards. RESULTS: After treatment indexes of the two groups were obviously improved, i. e. T, HR, RR, Paw, MV, the WBC, PCT, CRP were gradually declined, PaO(2)/FiO(2) was gradually raised. There were statistical significance before and 3 days after treatment in the trial and the control group [T: 37.35+/-0.91 centigrade vs. 38.43+/-1.06 centigrade, 37.28+/-0.88 centigrade vs. 38.35+/-1.11 centigrade; HR: 90.25+/-10.60 bpm vs. 118.94+/-15.46 bpm, 89.31+/-11.17 bpm vs. 124.34+/-17.87 bpm; RR: 25.14+/-3.17 bpm vs. 32.28+/-4.49 bpm, 24.81+/-2.43 bpm vs. 33.13+/-4.17 bpm; Paw: 23.03+/-3.04 cm H(2)O vs. 33.22+/-4.59 cm H(2)O, 22.75+/-3.22 cm H(2)O vs. 33.63+/-4.79 cm H(2)O; MV: 8.67+/-1.26 L/min vs. 11.80+/-2.01 L/min, 8.88+/-1.45 L/min vs. 13.21+/-2.90 L/min; WBC: 11.26+/-1.96 x10(9)/L vs. 14.57+/-3.10 x10(9)/L, 12.28+/-3.38 x10(9)/L vs. 15.25+/-4.93 x10(9)/L; PCT: 6.90+/-5.46 mug/L vs. 16.97+/-7.93 mug/L, 6.17+/-6.13 mug/L vs. 21.26+/-11.54 mug/L; CRP: 85.50+/-37.91 mg/L vs. 120.17+/-45.47 mg/L, 94.31+/-38.51 mg/L vs. 142.34+/-53.57 mg/L; PaO(2)/ FiO(2): 182.06+/-40.88 mm Hg vs. 98.67+/-20.62 mm Hg, 184.09+/-43.78 mm Hg vs. 96.22+/-22.59 mm Hg, all P<0.05]. There was no significant change in SpO(2) before and after treatment in two groups. And the total clinical effective rate in trial and control group were 83.4% and 81.2%, adverse reaction rate were 13.9% and 9.4%, bacterial removal rate were 90.3% and 87.0% respectively, and there was no significant difference between the two groups (all P>0.05). CONCLUSION: Domestic imipenem Cilastatin sodium can effectively control severe aspiration pneumonia, and it is safe and effective antibiotics.
Transcatheter arterial embolization using imipenem/cilastatin sodium for tendinopathy and enthesopathy refractory to nonsurgical management.[Pubmed:23707086]
J Vasc Interv Radiol. 2013 Jun;24(6):787-92.
PURPOSE: To evaluate the feasibility and effects of transcatheter arterial embolization with imipenem/Cilastatin sodium (CS) to treat tendinopathy and enthesopathy that are refractory to traditional nonsurgical management. MATERIALS AND METHODS: Transcatheter arterial embolization with imipenem/CS as an embolic agent was performed in seven patients (five men; mean age, 51.7 y) with tendinopathy and enthesopathy (patellar tendinopathy, n = 1; rotator cuff tendinopathy, n = 2; plantar fasciitis, n = 1; lateral epicondylitis, n = 1; iliotibial band syndrome, n = 1; and Achilles insertion tendinopathy, n = 1). All patients had unrelenting pain at the site of tendinopathy and enthesopathy before the procedure. Technical success, adverse events, and changes in visual analog scale (VAS) scores were assessed. RESULTS: All procedures were technically successful, and no major adverse events developed. Compared with before the procedure, mean VAS scores were significantly decreased at 1 day, 1 week, and 1 and 4 months after the procedure (72.7 mm+/-9.9 vs 17.4 mm+/-18.5, 16.0 mm+/-18.1, 13.7 mm+/-7.3, and 9.7 mm+/-6.8, respectively; all P< .001). CONCLUSIONS: Transcatheter arterial embolization with imipenem/CS was feasible and effectively relieved unrelenting pain associated with tendinopathy and enthesopathy.
Spectrophotometric and chemometric methods for determination of imipenem, ciprofloxacin hydrochloride, dexamethasone sodium phosphate, paracetamol and cilastatin sodium in human urine.[Pubmed:26709018]
Spectrochim Acta A Mol Biomol Spectrosc. 2016 Mar 15;157:26-33.
New accurate, sensitive and selective spectrophotometric and chemometric methods were developed and subsequently validated for determination of Imipenem (IMP), ciprofloxacin hydrochloride (CIPRO), dexamethasone sodium phosphate (DEX), paracetamol (PAR) and Cilastatin sodium (CIL) in human urine. These methods include a new derivative ratio method, namely extended derivative ratio (EDR), principal component regression (PCR) and partial least-squares (PLS) methods. A novel EDR method was developed for the determination of these drugs, where each component in the mixture was determined by using a mixture of the other four components as divisor. Peak amplitudes were recorded at 293.0 nm, 284.0 nm, 276.0 nm, 257.0 nm and 221.0 nm within linear concentration ranges 3.00-45.00, 1.00-15.00, 4.00-40.00, 1.50-25.00 and 4.00-50.00 mug mL(-1) for IMP, CIPRO, DEX, PAR and CIL, respectively. PCR and PLS-2 models were established for simultaneous determination of the studied drugs in the range of 3.00-15.00, 1.00-13.00, 4.00-12.00, 1.50-9.50, and 4.00-12.00 mug mL(-1) for IMP, CIPRO, DEX, PAR and CIL, respectively, by using eighteen mixtures as calibration set and seven mixtures as validation set. The suggested methods were validated according to the International Conference of Harmonization (ICH) guidelines and the results revealed that they were accurate, precise and reproducible. The obtained results were statistically compared with those of the published methods and there was no significant difference.
Inhibition of brush border dipeptidase with cilastatin reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells.[Pubmed:15252165]
Nephrol Dial Transplant. 2004 Oct;19(10):2445-55.
BACKGROUND: Cilastatin reduces nephrotoxicity associated with cyclosporin A (CyA) in solid organ and bone marrow transplantation. This appears to be unrelated to changes in renal haemodynamics or CyA metabolism. How cilastatin induces this protection is unclear, but it could result from changes on accumulation of CyA proximal cells. METHODS: We investigated the effects of cilastatin on primary cultures of pig kidney proximal tubule epithelial cells (PTECs) treated with CyA and FK506. Cell membrane fluidity and membrane-bound cholesterol-rich raft (MBCR) distribution were evaluated by fluorescence microscopy, and CyA transport by radioimmunoassay. Changes in CyA- and FK506-induced apoptosis were also evaluated by electron and light microscopy, flow cytometry, and detection of cytoplasmic nucleosones by enzyme-linked immuosorbent assay. RESULTS: CyA caused a dose-dependent reduction of cell membrane fluidity, which was prevented by pre-treating PTECs with cilastatin. Cilastatin also inhibited CyA transport across membranes and reduced recovery of CyA in mitochondria and membrane-bound fractions from cilastatin-treated PTECs. This effect was not related to an altered distribution of MBCRs, which are essential for CyA transport. Cilastatin protected against CyA- and FK506-induced apoptosis. CONCLUSIONS: Prevention of CyA-induced reduction of cell membrane fluidity and inhibition of CyA transport are features of cilastatin's direct effects on PTECs. Unaltered distribution of MBCRs in the presence of cilastatin suggests that cilastatin binding to raft-bound dipeptidases, rather than MBCR modifications, causes interference with CyA transport. These results provide additional insight into the mechanisms and scope of cilastatin nephroprotection.
A continuous fluorometric assay for leukotriene D4 hydrolase.[Pubmed:10075814]
Anal Biochem. 1999 Mar 15;268(2):245-51.
A fluorogenic substrate for assay of leukotriene D4 hydrolase (LTDase; EC 3.4.13.19) has been prepared and evaluated, using enzyme purified from porcine kidney. The compound is based on internal quenching of the synthetic, fluorescent amino acid d, l-2-amino-3-(7-methoxy-4-coumaryl)propanoic acid (d,l-Amp) by a 2, 4-dinitrophenyl (DNP) group. The compound is epsilon-DNP-l-Lys-d-Amp which incorporates the D-isomer of Amp to exploit the unique ability among mammalian peptidases for LTDase to hydrolyze peptides containing a d-amino acid in the C-terminal position. epsilon-DNP-l-Lys-d-Amp was found to be an excellent substrate for LTDase, with Km value of 370 microoffUnder the conditions of assay, the substrate was without noticeable quenching effect on the fluorescence of the product (d-Amp) liberated by the action of LTDase. Using porcine kidney microvillar membranes, which contain a battery of peptidases, the specific inhibitor of LTDase, cilastatin, completely inhibited the breakdown of epsilon-DNP-l-Lys-d-Amp, indicating that the substrate is selective for LTDase.
Inhibition of the mammalian beta-lactamase renal dipeptidase (dehydropeptidase-I) by (Z)-2-(acylamino)-3-substituted-propenoic acids.[Pubmed:3495664]
J Med Chem. 1987 Jun;30(6):1074-90.
The title enzyme deactivates the potent carbapenem antibiotic imipenem in the kidney, producing low antibiotic levels in the urinary tract. A series of (Z)-2-(acylamino)-3-substituted-propenoic acids (3) are specific, competitive inhibitors of the enzyme capable of increasing the urinary concentration of imipenem in vivo. Many of the compounds were prepared in one step from an alpha-keto acid and a primary amide. The optimum R2 groups are 2,2-dimethyl, -dichloro, and -dibromocyclopropyl. With R2 = 2,2-dimethylcyclopropyl (DMCP), a wide variety of R3 groups including alkyl, oxa- and thiaalkyl, and alkyl groups containing acidic, basic, and neutral substituents give effective inhibitors with Ki values of 0.02-1 microM and a range of pharmacokinetic properties. By resolution of enantiomers and X-ray crystallography, the enzyme-inhibitory activity of the DMCP group was found to reside with the 1S isomer. The cysteinyl compound 176 (cilastatin, MK-0791) has the desired pharmacological properties and has been chosen for combination with imipenem.


