ValganciclovirAntiviral medication CAS# 88110-89-8 |
- Cidofovir
Catalog No.:BCC2546
CAS No.:113852-37-2
- Adefovir Dipivoxil
Catalog No.:BCC5025
CAS No.:142340-99-6
- Valganciclovir HCl
Catalog No.:BCC4745
CAS No.:175865-59-5
- CMX001
Catalog No.:BCC4106
CAS No.:444805-28-1
- Letermovir
Catalog No.:BCC1700
CAS No.:917389-32-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 88110-89-8 | SDF | Download SDF |
| PubChem ID | 492440 | Appearance | Powder |
| Formula | C11H15N5O5 | M.Wt | 297.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
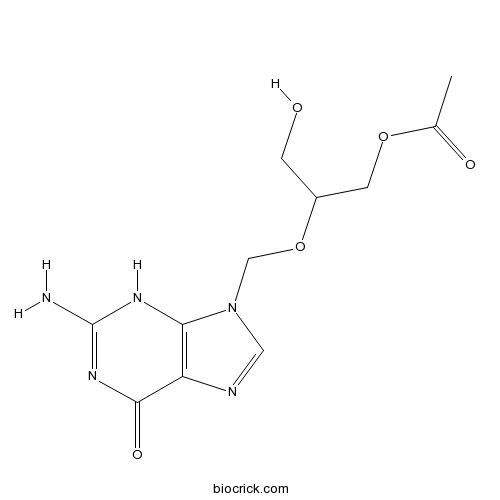
| Chemical Name | [2-[(2-amino-6-oxo-3H-purin-9-yl)methoxy]-3-hydroxypropyl] acetate | ||
| SMILES | CC(=O)OCC(CO)OCN1C=NC2=C1NC(=NC2=O)N | ||
| Standard InChIKey | YKLKCCHLLFMWQE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H15N5O5/c1-6(18)20-3-7(2-17)21-5-16-4-13-8-9(16)14-11(12)15-10(8)19/h4,7,17H,2-3,5H2,1H3,(H3,12,14,15,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Valganciclovir Dilution Calculator

Valganciclovir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3639 mL | 16.8197 mL | 33.6395 mL | 67.2789 mL | 84.0986 mL |
| 5 mM | 0.6728 mL | 3.3639 mL | 6.7279 mL | 13.4558 mL | 16.8197 mL |
| 10 mM | 0.3364 mL | 1.682 mL | 3.3639 mL | 6.7279 mL | 8.4099 mL |
| 50 mM | 0.0673 mL | 0.3364 mL | 0.6728 mL | 1.3456 mL | 1.682 mL |
| 100 mM | 0.0336 mL | 0.1682 mL | 0.3364 mL | 0.6728 mL | 0.841 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Valganciclovir is an antiviral medication used to treat cytomegalovirus infections. As the L-valyl ester of ganciclovir, it is actually an orally administered prodrug of the standard anti-cytomegalovirus (CMV) drug ganciclovir. After oral administration, Valganciclovir is rapidly converted to ganciclovir by intestinal and hepatic esterases.
- Notoginsenoside Fe
Catalog No.:BCN3852
CAS No.:88105-29-7
- 30-Hydroxygambogic acid
Catalog No.:BCN3081
CAS No.:881027-36-7
- HDS 029
Catalog No.:BCC7441
CAS No.:881001-19-0
- Notoginsenoside Fa
Catalog No.:BCN3854
CAS No.:88100-04-3
- Phrixotoxin 3
Catalog No.:BCC6328
CAS No.:880886-00-0
- 4-Hydroxybenzaldehyde rhamnoside
Catalog No.:BCN7625
CAS No.:88086-86-6
- PhiKan 083
Catalog No.:BCC2411
CAS No.:880813-36-5
- Naloxonazine dihydrochloride
Catalog No.:BCC6710
CAS No.:880759-65-9
- AMD-070 hydrochloride
Catalog No.:BCC1358
CAS No.:880549-30-4
- Fmoc-Hyp-OH
Catalog No.:BCC3254
CAS No.:88050-17-3
- Neuropathiazol
Catalog No.:BCC5375
CAS No.:880090-88-0
- 1,2-Benzenedicarboxylic acid
Catalog No.:BCN3151
CAS No.:88-99-3
- JNJ-26854165 (Serdemetan)
Catalog No.:BCC2240
CAS No.:881202-45-5
- Notoginsenoside Fc
Catalog No.:BCN3853
CAS No.:88122-52-5
- KU-0060648
Catalog No.:BCC1110
CAS No.:881375-00-4
- Daphniyunnine A
Catalog No.:BCN4428
CAS No.:881388-87-0
- Daphniyunnine B
Catalog No.:BCN4429
CAS No.:881388-88-1
- 6-Formyllimetin
Catalog No.:BCN3427
CAS No.:88140-31-2
- Dauriporphine
Catalog No.:BCN7903
CAS No.:88142-60-3
- DuP 697
Catalog No.:BCC7064
CAS No.:88149-94-4
- Amlodipine
Catalog No.:BCC4396
CAS No.:88150-42-9
- G-1
Catalog No.:BCC6045
CAS No.:881639-98-1
- 26RFa
Catalog No.:BCC6163
CAS No.:881640-56-8
- Levistilide A
Catalog No.:BCN1197
CAS No.:88182-33-6
A Physiologically Based Pharmacokinetic Model for Ganciclovir and Its Prodrug Valganciclovir in Adults and Children.[Pubmed:27450227]
AAPS J. 2016 Nov;18(6):1453-1463.
A physiologically based pharmacokinetic (PBPK) model has been developed for ganciclovir and its prodrug Valganciclovir. Initial bottom-up modeling based on physicochemical drug properties and measured in vitro inputs was verified in preclinical animal species, and then, a clinical model was verified in a stepwise fashion with pharmacokinetic data in adult, children, and neonatal patients. The final model incorporated conversion of Valganciclovir to ganciclovir through esterases and permeability-limited tissue distribution of both drugs with active transport processes added in gut, liver, and kidney. A PBPK model which accounted for known age-related tissue volumes, composition and blood flows, and renal filtration clearance was able to simulate well the measured plasma exposures in adults and pediatric patients. Overall, this work illustrates the stepwise development of PBPK models which could be used to predict pharmacokinetics in infants and neonates, thereby assisting drug development in a vulnerable patient population where clinical data are challenging to obtain.
Combined environmental risk assessment for the antiviral pharmaceuticals ganciclovir and valganciclovir in Europe.[Pubmed:28198039]
Environ Toxicol Chem. 2017 Aug;36(8):2205-2216.
Potential environmental risks of the old antiviral pharmaceuticals ganciclovir (GCV) and Valganciclovir (VGCV) were reassessed based on new environmental fate and chronic ecotoxicity tests and on actual use data for Europe. Valganciclovir is hydrolyzed to GCV by intestinal and hepatic esterases, and hence the new environmental tests only refer to GCV. A sorption study showed that GCV will not sorb significantly, excluding the soil as a relevant environmental compartment. Despite earlier data suggesting nondegradability, a new water/sediment fate test showed GCV to be primarily and ultimately degraded and to be nonpersistent. The chronic ecotoxicity tests with algae and daphnids resulted in no inhibition at the highest tested concentrations, whereas a fish partial life cycle test, selected in view of mammalian mutagenicity and reprotoxicity data, showed effects on growth of the young fish, but not on gametogenesis, fertilization, embryogenesis, or teratogenicity. Predicted environmental concentrations were derived based on actual per capita use data for European countries for 2004 to 2014, and the highest was selected for the risk assessment. A comparison of predicted environmental concentrations with predicted no-effect concentrations shows no significant risk for wastewater treatment, surface waters, groundwater, or sediment. In addition, potential risks to (semi)aquatic top predators or to human consumers of water and fish are exceedingly low. Environ Toxicol Chem 2017;36:2205-2216. (c) 2017 The Author. Environmental Toxicology and Chemistry Published by Wiley Periodicals, Inc. on behalf of SETAC.
Effect of Cytomegalovirus Infection on Survival of Older Kidney Transplant Patients (D+/R+): Impact of Valganciclovir Prophylaxis Versus Preemptive Therapy.[Pubmed:27932110]
Transplant Proc. 2016 Nov;48(9):2931-2937.
BACKGROUND: Kidney transplant patients with D+/R+ serology can be treated with either prophylaxis or preemptive Valganciclovir. The older transplant population suffers severe immunosenescence, especially patients with latent cytomegalovirus (CMV) infection (R+). They are more likely to develop indirect CMV effects. Likewise, many patients have significant cardiovascular comorbidity, which makes them more sensitive to these indirect effects. The aim of this study was to evaluate the incidence of CMV viremia and indirect effects on survival, comparing prophylaxis (V) against preemptive (P) Valganciclovir in an older kidney transplant population. METHODS: We analyzed the data of 233 recipients from 2002 (age, >55 years; D+/R+) with >/=6 months of follow-up. The patients were divided into 2 groups: 167 (71.7%) in the V group and 66 (28.3%) in the P group. RESULTS: The incidence of CMV infection in the P group was 32% versus 6% in V group. Patients with CMV viremia showed worse survival values than patients without viremia (log rank P = .031). Five-year survivals were 74% vs 88%, respectively. Cox regression showed that the adjusted effect of CMV infection on overall survival was a significant risk (hazard ratio [HR], 2.07; 95% CI, 1.003-4.29). Patients with CMV viremia showed worse cardiovascular survival than patients without viremia, with 5-year survivals of 79% vs 94%. Cox regression showed that the adjusted effect of CMV infection was a significant risk (HR, 2.62). CONCLUSIONS: CMV infection has a detrimental effect on the survival of older patients. Valganciclovir prophylaxis induces a protective effect against CMV infection and could improve survival of older patients with cardiovascular comorbidities.
Successful Cost-Effective Prevention of Cytomegalovirus Disease in Kidney Transplant Recipients Using Low-Dose Valganciclovir.[Pubmed:28260458]
Exp Clin Transplant. 2017 Feb;15(Suppl 1):156-163.
OBJECTIVES: Low-dose Valganciclovir prophylaxis is still under investigation in renal transplant procedures. Our aim was to assess the cost effectiveness of 450 mg versus 900 mg Valganciclovir prophylaxis in kidney transplant recipients. MATERIALS AND METHODS: In this prospective trial, 201 kidney transplant patients were randomized (1:1) to receive 450 mg/d (group 1, n = 100) or 900 mg/d (group 2, n = 101) Valganciclovir prophylaxis for the first 6 months after transplant. Patients were studied for incidence of cytomegalovirus disease, leucopenia episodes, rejection episodes, and graft outcomes along with associated costs over 1 year. Costs (in US dollars) of treatment of rejection were also analyzed. RESULTS: Demographic features of the studied groups were comparable. We found that the cost of cytomegalovirus care in group 1 patients was significantly lower (by 50% at 6 months; P < .001), with less leukopenia episodes (P = .04), lower doses of granulocyte colony-stimulating factor (by 30% at 6 months; P = .03), higher doses of mycophenolate mofetil (P = .04), and less rejection episodes (P = .01) compared with group 2. In group 2, there were more episodes of cytomegalovirus infection (P = .052) and BK virus nephropathy (P = .04). Graft and patient outcomes were satisfactory in both groups. CONCLUSIONS: Low-dose Valganciclovir for cytomegalovirus prophylaxis after renal transplant is safer, effective and without breakthrough infection, and less costly than using the usual dose.


