BMS 191011maxi-K channel opener CAS# 202821-81-6 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- Iniparib (BSI-201)
Catalog No.:BCC2208
CAS No.:160003-66-7
- AG-14361
Catalog No.:BCC2209
CAS No.:328543-09-5
- PJ34 hydrochloride
Catalog No.:BCC2210
CAS No.:344458-15-7
- Rucaparib (AG-014699,PF-01367338)
Catalog No.:BCC2207
CAS No.:459868-92-9
- Veliparib dihydrochloride
Catalog No.:BCC2076
CAS No.:912445-05-7
Quality Control & MSDS
Number of papers citing our products

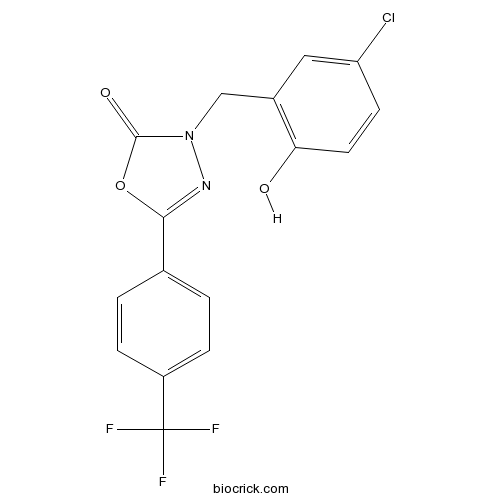
Chemical structure

3D structure
| Cas No. | 202821-81-6 | SDF | Download SDF |
| PubChem ID | 10474339 | Appearance | Powder |
| Formula | C16H10ClF3N2O3 | M.Wt | 370.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (337.19 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 3-[(5-chloro-2-hydroxyphenyl)methyl]-5-[4-(trifluoromethyl)phenyl]-1,3,4-oxadiazol-2-one | ||
| SMILES | C1=CC(=CC=C1C2=NN(C(=O)O2)CC3=C(C=CC(=C3)Cl)O)C(F)(F)F | ||
| Standard InChIKey | QKOWACXSXTXRKA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H10ClF3N2O3/c17-12-5-6-13(23)10(7-12)8-22-15(24)25-14(21-22)9-1-3-11(4-2-9)16(18,19)20/h1-7,23H,8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent BKCa channel opener (large-conductance Ca2+-activated potassium channel, KCa1.1). Neuroprotectant in two distinct animal models of stroke (MCAO in the SHR rat and a normotensive model of focal stroke). |

BMS 191011 Dilution Calculator

BMS 191011 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6975 mL | 13.4876 mL | 26.9753 mL | 53.9505 mL | 67.4382 mL |
| 5 mM | 0.5395 mL | 2.6975 mL | 5.3951 mL | 10.7901 mL | 13.4876 mL |
| 10 mM | 0.2698 mL | 1.3488 mL | 2.6975 mL | 5.3951 mL | 6.7438 mL |
| 50 mM | 0.054 mL | 0.2698 mL | 0.5395 mL | 1.079 mL | 1.3488 mL |
| 100 mM | 0.027 mL | 0.1349 mL | 0.2698 mL | 0.5395 mL | 0.6744 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BMS 191011 is a maxi-K channel opener [1].
Maxi-K channels consist of a pore-forming α subunit and a regulatory β subunit. Maxi-K channels are of a high Ca2+ sensitivity [2].
Bath application of BMS-191011 at a concentration of 20 μM strongly reduced the calcium transients. This effect was associated with bursts of bAPs (100 Hz) recorded from Fmr1-/y dendrites without affecting those recorded from wild-type dendrites. This treatment decreased dendritic calcium transients of Fmr1-/y neurons to baseline levels of wild-type neurons [3]. In normoxia, BMS-191011 significantly induced cell death. This effect was indicted by the increases in propidium iodide (PI) uptake by 9.4 ± 2.4 and 16.8 ± 2.1% at 12 and 24 h treatments, respectively. At 12 h and then 24 h, the cellular [ATP] was decreased to 83.4 ± 3.1 and further to 72.3 ± 2.8%. During hypoxia, these effects were increased by ~2-fold in all time points and measurements. PI uptake was increased to 15.1 ± 1.8 at 12 h and then 40.7 ± 1.7% at 24 h. Cellular [ATP] was decreased to 77.8 ± 1.9 at 12 h and then to 43.3 ± 3.4% at 24 h [4].
In male Wistar rats of 8 to 10 weeks old, an i.v. administration with BMS-191011 at 10-100 µg/kg/min increased the retinal arteriol diameter, whereas it did not significantly affect mean arterial pressure and heart rate. Intravitreal injection of iberiotoxin at a dose of 20 pmol/eye significantly attenuated the vasodilator responses of retinal arterioles to BMS-191011 [5]. BMS-191011 demonstrated efficacy as an opener of the cloned large-conductance Ca2+-activated potassium (maxi-K) channel in in vivo stroke models [6].
References:
[1]. Hewawasam P, Ding M, Chen N, et al. Synthesis of water-soluble prodrugs of BMS-191011: a maxi-K channel opener targeted for post-stroke neuroprotection. Bioorganic & medicinal chemistry letters, 2003, 13(10): 1695-1698.
[2]. Valverde MA, Rojas P, Amigo J, et al. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science, 1999, 285(5435): 1929-1931.
[3]. Zhang Y, Bonnan A, Bony G, et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1-/y mice. Nature neuroscience, 2014, 17(12): 1701-1709.
[4]. Gu XQ, Pamenter ME, Siemen D, et al. Mitochondrial but not plasmalemmal BK channels are hypoxia-sensitive in human glioma. Glia, 2014, 62(4): 504-513.
[5]. Mori A, Suzuki S, Sakamoto K, et al. BMS-191011, an opener of large-conductance Ca2+-activated potassium channels, dilates rat retinal arterioles in vivo. Biological and Pharmaceutical Bulletin, 2011, 34(1): 150-152.
[6]. Romine JL, Martin SW, Meanwell NA, et al. 3-[(5-Chloro-2-hydroxyphenyl) methyl]-5-[4-(trifluoromethyl) phenyl]-1, 3, 4-oxadiazol-2 (3 H)-one, BMS-191011: Opener of Large-Conductance Ca2+-Activated Potassium (Maxi-K) Channels, Identification, Solubility, and SAR. Journal of medicinal chemistry, 2007, 50(3): 528-542.
- Licoagrochalcone A
Catalog No.:BCC8197
CAS No.:202815-28-9
- Orexin B (mouse)
Catalog No.:BCC5766
CAS No.:202801-92-1
- 4-Methoxycoumarine
Catalog No.:BCN6536
CAS No.:20280-81-3
- Homodihydrocapsaicin I
Catalog No.:BCN7844
CAS No.:20279-06-5
- Forsythenside A
Catalog No.:BCN6440
CAS No.:202721-09-3
- Nicotinamide N-oxide
Catalog No.:BCN1969
CAS No.:1986-81-8
- 3α-Bis-(4-fluorophenyl) methoxytropane hydrochloride
Catalog No.:BCC6846
CAS No.:202646-03-5
- JHW 007 hydrochloride
Catalog No.:BCC7923
CAS No.:202645-74-7
- DL-AP4
Catalog No.:BCC6548
CAS No.:20263-07-4
- DL-AP3
Catalog No.:BCC2459
CAS No.:20263-06-3
- Ginkgolic acid C13:0
Catalog No.:BCN5333
CAS No.:20261-38-5
- H-Valinol
Catalog No.:BCC2696
CAS No.:2026-48-4
- Ralfinamide mesylate
Catalog No.:BCC7844
CAS No.:202825-45-4
- Safinamide Mesylate
Catalog No.:BCC2320
CAS No.:202825-46-5
- Rosmarinic acid
Catalog No.:BCN5893
CAS No.:20283-92-5
- 8-Hydroxy-3,5,7,3',4',5'-hexamethoxyflavone
Catalog No.:BCN1506
CAS No.:202846-95-5
- 4-Benzyloxyindole
Catalog No.:BCC8700
CAS No.:20289-26-3
- 7-Benzyloxyindole
Catalog No.:BCC8778
CAS No.:20289-27-4
- glucagon receptor antagonists 3
Catalog No.:BCC1595
CAS No.:202917-17-7
- glucagon receptor antagonists 2
Catalog No.:BCC1594
CAS No.:202917-18-8
- Conantokin-R
Catalog No.:BCC5980
CAS No.:202925-60-8
- NF 340
Catalog No.:BCC7785
CAS No.:202982-98-7
- NF 279
Catalog No.:BCC6964
CAS No.:202983-32-2
- Aporheine
Catalog No.:BCN4802
CAS No.:2030-53-7
BMS-191011, an opener of large-conductance Ca2+-activated potassium channels, dilates rat retinal arterioles in vivo.[Pubmed:21212534]
Biol Pharm Bull. 2011;34(1):150-2.
The large-conductance Ca(2+)-activated K(+) (BK(Ca)) channels modulate vascular smooth muscle tone but the role of BK(Ca) channels in regulation of retinal circulation remains unclear. In the present study, we examined the effects of BMS-191011 and NS 1619, openers of BK(Ca) channels, on rat retinal blood vessels in vivo. Male Wistar rats (8- to 10-week-old) were anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneally (i.p.)) and treated with tetrodotoxin (50 microg/kg, intravenously (i.v.)) to eliminate any nerve activity and prevent movement of the eye under artificial ventilation. A mixture solution of adrenaline and noradrenaline (9:1) was infused to maintain adequate systemic circulation. BMS-191011 (10-100 microg/kg, i.v.) and NS 1619 (0.1-1.0 microg/kg, i.v.) increased the diameter of retinal arterioles without altering systemic blood pressure and heart rate significantly. The vasodilator responses to BMS-191011, but not to NS 1619, were significantly diminished by intravitreal injection of iberiotoxin (an inhibitor of BK(Ca) channels, 20 pmol/eye). These results suggest that BMS-191011 dilates rat retinal arterioles through activation of iberiotoxin-sensitive BK(Ca) channels in vivo. The BK(Ca) channel opener could be considered as a candidate for improving retinal circulation without severe cardiovascular side-effects.
3-[(5-Chloro-2-hydroxyphenyl)methyl]-5-[4-(trifluoromethyl)phenyl ]-1,3,4-oxadiazol-2(3H)-one, BMS-191011: opener of large-conductance Ca(2+)-activated potassium (maxi-K) channels, identification, solubility, and SAR.[Pubmed:17266205]
J Med Chem. 2007 Feb 8;50(3):528-42.
Compound 8a (BMS-191011), an opener of the cloned large-conductance, Ca2+-activated potassium (maxi-K) channel, demonstrated efficacy in in vivo stroke models, which led to its nomination as a candidate for clinical evaluation. Its maxi-K channel opening properties were consistent with its structural topology, being derived by combining elements from other known maxi-K openers. However, 8a suffered from poor aqueous solubility, which complicated elucidation of SAR during in vitro evaluation. The activity of 8a in in vivo stroke models and studies directed toward improving its solubility are reported herein. Enhanced solubility was achieved by appending heterocycles to the 8a scaffold, and a notable observation was made that inclusion of a simple amino group (anilines 8k and 8l) yielded excellent in vitro maxi-K ion channel opening activity and enhanced brain-to-plasma partitioning compared to the appended heterocycles.
Synthesis of water-soluble prodrugs of BMS-191011: a maxi-K channel opener targeted for post-stroke neuroprotection.[Pubmed:12729644]
Bioorg Med Chem Lett. 2003 May 19;13(10):1695-8.
A variety of water-soluble prodrugs of BMS-191011 was synthesized and evaluated for solution state stability and rate of conversion to BMS-191011 in rat and human plasma. The deoxycarnitine ester prodrug (11c) was selected for clinical evaluation based on its superior chemical stability, crystallinity and cleavage to BMS-191011 in human plasma.


