Rucaparib (AG-014699,PF-01367338)Potent PARP inhibitor CAS# 459868-92-9 |
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- ME0328
Catalog No.:BCC3995
CAS No.:1445251-22-8
- Rucaparib (free base)
Catalog No.:BCC4012
CAS No.:283173-50-2
- PJ34 hydrochloride
Catalog No.:BCC2210
CAS No.:344458-15-7
- Olaparib (AZD2281, Ku-0059436)
Catalog No.:BCC2206
CAS No.:763113-22-0
- NU 1025
Catalog No.:BCC2454
CAS No.:90417-38-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure
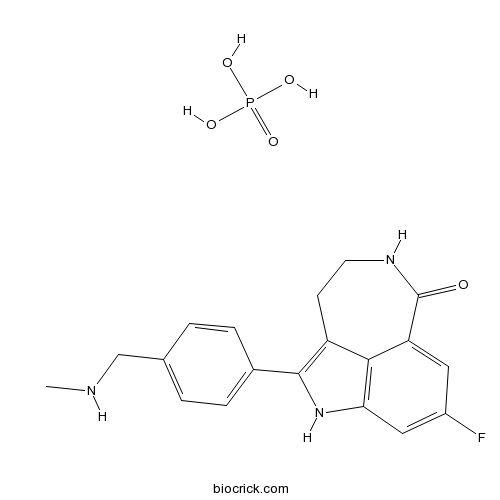
3D structure
| Cas No. | 459868-92-9 | SDF | Download SDF |
| PubChem ID | 9931953 | Appearance | Powder |
| Formula | C19H21FN3O5P | M.Wt | 421.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AG-014699 phosphate; PF-01367338 phosphate | ||
| Solubility | DMSO : ≥ 33 mg/mL (78.32 mM) *"≥" means soluble, but saturation unknown. | ||
| SMILES | CNCC1=CC=C(C=C1)C2=C3CCNC(=O)C4=CC(=CC(=C34)N2)F.OP(=O)(O)O | ||
| Standard InChIKey | FCCGJTKEKXUBFZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18FN3O.H3O4P/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15;1-5(2,3)4/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24);(H3,1,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Rucaparib (AG-014699, PF-01367338) is an inhibitor of PARP1 with Ki of 1.4 nM. | |||||
| Targets | PARP | |||||
| IC50 | 1.4 nM (Ki) | |||||
| Cell experiment [1]: | |
| Cell lines | Canine kidney MDCKII cell lines |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 8h; 5 μM |
| Applications | In the MDCKII parental cell line, which overexpressed human (h) ABCB1, both apically and basolaterally directed translocation of rucaparib were the same. Treatment of the cells with the ABCB1 inhibitor zosuquidar resulted in a slight decrease in apically directed transport, which could be either due to a specific inhibition of an unidentified rucaparib uptake transporter at the basolateral side, or inhibition of endogenous canine ABCB1. The result shown that rucaparib is a transported substrate of ABCB1. |
| Animal experiment [1]: | |
| Animal models | female WT, Abcb1a/1b mice of a >99% FVB genetic background |
| Dosage form | 10 mg/kg; oral taken |
| Application | We analyzed the separate and combined effect of Abcg2 and Abcb1a/1b activity on the in vivo disposition of orally administered rucaparib at a dose of 10 mg/kg in wild-type (WT) and single and combination Abcg2 and Abcb1a/1b knockout mice. In vivo, oral availability (plasma AUC0-1 and AUC0-24) and brain levels of rucaparib at 1 and 24 h were increased by the absence of both Abcg2 and Abcb1a/1b after oral administration of rucaparib at 10 mg/kg. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Durmus S, Sparidans R W, van Esch A, et al. Breast Cancer Resistance Protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1) Restrict Oral Availability and Brain Accumulation of the PARP Inhibitor Rucaparib (AG-014699)[J]. Pharmaceutical research, 2014: 1-10. | |

Rucaparib (AG-014699,PF-01367338) Dilution Calculator

Rucaparib (AG-014699,PF-01367338) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3733 mL | 11.8663 mL | 23.7327 mL | 47.4654 mL | 59.3317 mL |
| 5 mM | 0.4747 mL | 2.3733 mL | 4.7465 mL | 9.4931 mL | 11.8663 mL |
| 10 mM | 0.2373 mL | 1.1866 mL | 2.3733 mL | 4.7465 mL | 5.9332 mL |
| 50 mM | 0.0475 mL | 0.2373 mL | 0.4747 mL | 0.9493 mL | 1.1866 mL |
| 100 mM | 0.0237 mL | 0.1187 mL | 0.2373 mL | 0.4747 mL | 0.5933 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Rucaparib, also named as AG-014699 or PF-01367338, is a poly (ADP ribose) polymerase (PARP) inhibitor. PARP is a DNA damage-activated nuclear enzyme that has a key signaling role in the base excision repair pathway. So, rucaparib has been also found to be most effective in cells deficient in DNA repair, where the cells deficient are caused by exposure to genotoxic agents, such as irradiation produces DNA damage and its toxicity is augmented when the DNA repair is impaired. Increased radiosensitivity in presence of rucaparib was associated with persistent DNA breaks as determined by gamma-H2AX and p53BP1 foci. Rucaparib radiosensitizes prostate cancer cells, most effectively those that are PTEN-deficient and are expressing ETS gene fusion proteins, which inhibits NHEJ DNA repair.
References
Ruth Plummer, Paul Lorigan, Neil Steven, Lucy Scott, Mark R. Middleton, Richard H. Wilson, Evan Mulligan, Nicola Curtin, Diane Wang, Raz Dewji, Antonello Abbattista, Jorge Gallo, Hilary Calvert. A phase II study of the potent PARP inhibitor, Rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation.
Payel Chatterjee, Gaurav Choudhary, Warren D. Heston, Eric A. Klein, Alex Almasan. The PARP inhibitor rucaparib radiosensitizes prostate cancer cells, most effectively those that are PTEN-deficient and are expressing ETS gene fusion proteins, which inhibit NHEJ DNA repair. Cancer Research. 2012. 72: B27.
- 5-[(2R)-2-Aminopropyl]-2,3-dihydro-1-[3-(phenylmethoxy)propyl]-1H-indole-7-carbonitrile
Catalog No.:BCN1438
CAS No.:459868-73-6
- PEAQX
Catalog No.:BCC5495
CAS No.:459836-30-7
- Obeticholic Acid
Catalog No.:BCC5572
CAS No.:459789-99-2
- SB 452533
Catalog No.:BCC7620
CAS No.:459429-39-1
- JNJ-7777120
Catalog No.:BCC4543
CAS No.:459168-41-3
- SW033291
Catalog No.:BCC3981
CAS No.:459147-39-8
- Boc-Asn-ONp
Catalog No.:BCC3072
CAS No.:4587-33-1
- Ilicic acid
Catalog No.:BCN5505
CAS No.:4586-68-9
- Curcumin
Catalog No.:BCN5504
CAS No.:458-37-7
-
Scutebarbatine L
Catalog No.:BCN8369
CAS No.:960302-91-4
- BMS 470539 dihydrochloride
Catalog No.:BCC7850
CAS No.:457893-92-4
- PA 452
Catalog No.:BCC8005
CAS No.:457657-34-0
- Interiotherin C
Catalog No.:BCN3636
CAS No.:460090-65-7
- Larixyl acetate
Catalog No.:BCC8195
CAS No.:4608-49-5
- Eact
Catalog No.:BCC6313
CAS No.:461000-66-8
- Ko 143
Catalog No.:BCC1684
CAS No.:461054-93-3
- Dapagliflozin
Catalog No.:BCC2552
CAS No.:461432-26-8
- 4beta,12-dihydroxyguaian-6,10-diene
Catalog No.:BCN7829
CAS No.:461644-90-6
- Lactulose
Catalog No.:BCC4669
CAS No.:4618-18-2
- Gnemonol B
Catalog No.:BCN3399
CAS No.:462636-74-4
- alpha-Linolenic acid
Catalog No.:BCN8319
CAS No.:463-40-1
- Bay 55-9837
Catalog No.:BCC5932
CAS No.:463930-25-8
- (+)-Borneol
Catalog No.:BCC8376
CAS No.:464-43-7
- (-)-Borneol
Catalog No.:BCC8897
CAS No.:464-45-9
Targeted therapy for ovarian cancer: the rapidly evolving landscape of PARP inhibitor use.[Pubmed:28994564]
Minerva Ginecol. 2018 Apr;70(2):150-170.
INTRODUCTION: Poly(ADP-ribose) polymerase (PARP) inhibitors are a targeted therapy option for ovarian cancer. The goal of this review was to organize and summarize the clinical trials evaluating PARP inhibitor therapy in ovarian cancer as monotherapy, maintenance therapy after partial or complete remission to therapy or as a part of a combination regimen. EVIDENCE ACQUISITION: PubMed, ClinicalTrials.gov, data from the United States Food and Drug Administration (US FDA) and proceedings from scientific conferences were searched for published and unpublished data pertaining to clinical trials and approvals of PARP inhibitor use in ovarian cancer. EVIDENCE SYNTHESIS: There have been 36 published phase 1, 2 and 3 studies evaluating the use of olaparib, niraparib, veliparib and rucaparib in ovarian cancer. Olaparib and rucaparib have been approved by the US FDA as monotherapy for advanced recurrent ovarian cancer. Niraparib and olaparib have been approved by the US FDA for maintenance therapy after partial or complete remission in recurrent ovarian cancer. There are currently ten phase 3 trials evaluating PARP inhibitors at various timepoints in ovarian cancer therapy including at the time of primary adjuvant therapy, as maintenance therapy after primary chemotherapy, as monotherapy for recurrent cancer and as maintenance therapy after chemotherapy for recurrence. CONCLUSIONS: The landscape of PARP inhibitor use for ovarian cancer is rapidly evolving and PARP inhibitors have become more available as a targeted therapy option for ovarian cancer treatment.
Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial.[Pubmed:28916367]
Lancet. 2017 Oct 28;390(10106):1949-1961.
BACKGROUND: Rucaparib, a poly(ADP-ribose) polymerase inhibitor, has anticancer activity in recurrent ovarian carcinoma harbouring a BRCA mutation or high percentage of genome-wide loss of heterozygosity. In this trial we assessed rucaparib versus placebo after response to second-line or later platinum-based chemotherapy in patients with high-grade, recurrent, platinum-sensitive ovarian carcinoma. METHODS: In this randomised, double-blind, placebo-controlled, phase 3 trial, we recruited patients from 87 hospitals and cancer centres across 11 countries. Eligible patients were aged 18 years or older, had a platinum-sensitive, high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube carcinoma, had received at least two previous platinum-based chemotherapy regimens, had achieved complete or partial response to their last platinum-based regimen, had a cancer antigen 125 concentration of less than the upper limit of normal, had a performance status of 0-1, and had adequate organ function. Patients were ineligible if they had symptomatic or untreated central nervous system metastases, had received anticancer therapy 14 days or fewer before starting the study, or had received previous treatment with a poly(ADP-ribose) polymerase inhibitor. We randomly allocated patients 2:1 to receive oral rucaparib 600 mg twice daily or placebo in 28 day cycles using a computer-generated sequence (block size of six, stratified by homologous recombination repair gene mutation status, progression-free interval after the penultimate platinum-based regimen, and best response to the most recent platinum-based regimen). Patients, investigators, site staff, assessors, and the funder were masked to assignments. The primary outcome was investigator-assessed progression-free survival evaluated with use of an ordered step-down procedure for three nested cohorts: patients with BRCA mutations (carcinoma associated with deleterious germline or somatic BRCA mutations), patients with homologous recombination deficiencies (BRCA mutant or BRCA wild-type and high loss of heterozygosity), and the intention-to-treat population, assessed at screening and every 12 weeks thereafter. This trial is registered with ClinicalTrials.gov, number NCT01968213; enrolment is complete. FINDINGS: Between April 7, 2014, and July 19, 2016, we randomly allocated 564 patients: 375 (66%) to rucaparib and 189 (34%) to placebo. Median progression-free survival in patients with a BRCA-mutant carcinoma was 16.6 months (95% CI 13.4-22.9; 130 [35%] patients) in the rucaparib group versus 5.4 months (3.4-6.7; 66 [35%] patients) in the placebo group (hazard ratio 0.23 [95% CI 0.16-0.34]; p<0.0001). In patients with a homologous recombination deficient carcinoma (236 [63%] vs 118 [62%]), it was 13.6 months (10.9-16.2) versus 5.4 months (5.1-5.6; 0.32 [0.24-0.42]; p<0.0001). In the intention-to-treat population, it was 10.8 months (8.3-11.4) versus 5.4 months (5.3-5.5; 0.36 [0.30-0.45]; p<0.0001). Treatment-emergent adverse events of grade 3 or higher in the safety population (372 [99%] patients in the rucaparib group vs 189 [100%] in the placebo group) were reported in 209 (56%) patients in the rucaparib group versus 28 (15%) in the placebo group, the most common of which were anaemia or decreased haemoglobin concentration (70 [19%] vs one [1%]) and increased alanine or aspartate aminotransferase concentration (39 [10%] vs none). INTERPRETATION: Across all primary analysis groups, rucaparib significantly improved progression-free survival in patients with platinum-sensitive ovarian cancer who had achieved a response to platinum-based chemotherapy. ARIEL3 provides further evidence that use of a poly(ADP-ribose) polymerase inhibitor in the maintenance treatment setting versus placebo could be considered a new standard of care for women with platinum-sensitive ovarian cancer following a complete or partial response to second-line or later platinum-based chemotherapy. FUNDING: Clovis Oncology.
Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2.[Pubmed:28882436]
Gynecol Oncol. 2017 Nov;147(2):267-275.
OBJECTIVE: An integrated analysis was undertaken to characterize the antitumor activity and safety profile of the oral poly(ADP-ribose) polymerase inhibitor rucaparib in patients with relapsed high-grade ovarian carcinoma (HGOC). METHODS: Eligible patients from Study 10 (NCT01482715) and ARIEL2 (NCT01891344) who received a starting dose of oral rucaparib 600mg twice daily (BID) with or without food were included in these analyses. The integrated efficacy population included patients with HGOC and a deleterious germline or somatic BRCA1 or BRCA2 (BRCA1/2) mutation who received at least two prior chemotherapies and were sensitive, resistant, or refractory to platinum-based chemotherapy. The primary endpoint was investigator-assessed confirmed objective response rate (ORR). Secondary endpoints included duration of response (DOR) and progression-free survival (PFS). The integrated safety population included patients with HGOC who received at least one dose of rucaparib 600mg BID, irrespective of BRCA1/2 mutation status and prior treatments. RESULTS: In the efficacy population (n=106), ORR was 53.8% (95% confidence interval [CI], 43.8-63.5); 8.5% and 45.3% of patients achieved complete and partial responses, respectively. Median DOR was 9.2months (95% CI, 6.6-11.6). In the safety population (n=377), the most frequent treatment-emergent adverse events (AEs) were nausea, asthenia/fatigue, vomiting, and anemia/hemoglobin decreased. The most common grade >/=3 treatment-emergent AE was anemia/hemoglobin decreased. Treatment-emergent AEs led to treatment interruption, dose reduction, and treatment discontinuation in 58.6%, 45.9%, and 9.8% of patients, respectively. No treatment-related deaths occurred. CONCLUSIONS: Rucaparib has antitumor activity in advanced BRCA1/2-mutated HGOC and a manageable safety profile.
Targeted therapy of ovarian cancer including immune check point inhibitor.[Pubmed:28823141]
Korean J Intern Med. 2017 Sep;32(5):798-804.
Epithelial ovarian cancer is the eighth most common cause of cancer-related deaths in women because most patients present with advanced stage disease at the time of diagnosis. Although cytoreductive surgery and platinum-based chemotherapy remain the gold standards of treatment, the recurrence rate of ovarian cancer remains high. Attempts to improve this standard two-drug chemotherapy by adding a third cytotoxic drug have failed to affect either progression-free survival or overall survival and have resulted in an increase in toxic side effects. Some anti-angiogenic agents, poly(ADP-ribose) polymerase, and immune checkpoint inhibitors have shown efficacy in early stages of development for the treatment of epithelial ovarian cancer. As demonstrated in recent clinical trials, the use of bevacizumab, cediranib, pazopanib, olaparib, and rucaparib, either alone or in combination with conventional cytotoxic agents, improves progression-free survival. Trials on immune checkpoint inhibitors such as nivolumab have revealed prolonged responses in a small set of ovarian cancer cases but require further exploration. In this review, we discuss the role of targeted therapies against ovarian cancer, including the use of immune checkpoint inhibitors.
The HRD Decision-Which PARP Inhibitor to Use for Whom and When.[Pubmed:28974545]
Clin Cancer Res. 2017 Dec 1;23(23):7155-7157.
Rucaparib, a polyADPribose polymerase inhibitor (PARPi), was approved recently for use in women with high-grade serous ovarian cancer (HGSOC). It is now one of three approved PARPi for use in recurrent ovarian cancer, a family of agents that has changed the HGSOC treatment landscape and outcome. Clin Cancer Res; 23(23); 7155-7. (c)2017 AACRSee related article by Balasubramaniam et al., p. 7165.


