Ac-Trp-OEtCAS# 2382-80-1 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 2382-80-1 | SDF | Download SDF |
| PubChem ID | 75423 | Appearance | Powder |
| Formula | C15H18N2O3 | M.Wt | 274.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
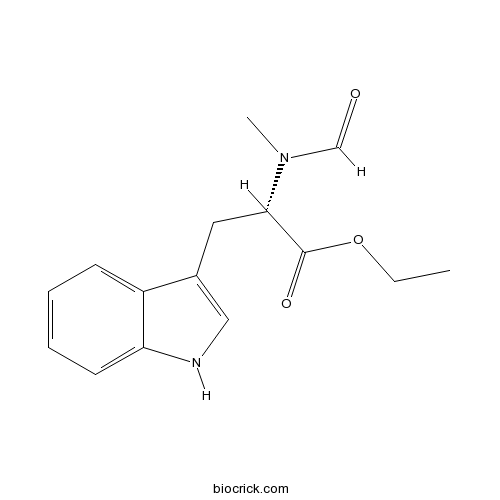
| Chemical Name | ethyl (2S)-2-[formyl(methyl)amino]-3-(1H-indol-3-yl)propanoate | ||
| SMILES | CCOC(=O)C(CC1=CNC2=CC=CC=C21)N(C)C=O | ||
| Standard InChIKey | LYXGYFZZVMODTB-AWEZNQCLSA-N | ||
| Standard InChI | InChI=1S/C15H18N2O3/c1-3-20-15(19)14(17(2)10-18)8-11-9-16-13-7-5-4-6-12(11)13/h4-7,9-10,14,16H,3,8H2,1-2H3/t14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ac-Trp-OEt Dilution Calculator

Ac-Trp-OEt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6456 mL | 18.2282 mL | 36.4564 mL | 72.9129 mL | 91.1411 mL |
| 5 mM | 0.7291 mL | 3.6456 mL | 7.2913 mL | 14.5826 mL | 18.2282 mL |
| 10 mM | 0.3646 mL | 1.8228 mL | 3.6456 mL | 7.2913 mL | 9.1141 mL |
| 50 mM | 0.0729 mL | 0.3646 mL | 0.7291 mL | 1.4583 mL | 1.8228 mL |
| 100 mM | 0.0365 mL | 0.1823 mL | 0.3646 mL | 0.7291 mL | 0.9114 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ac-Trp-OEt
- Trichokaurin
Catalog No.:BCN4851
CAS No.:23811-50-9
- Hinesol
Catalog No.:BCC9232
CAS No.:23811-08-7
- Pogostone
Catalog No.:BCN2696
CAS No.:23800-56-8
- 6-Hydroxyindole
Catalog No.:BCN8310
CAS No.:2380-86-1
- Homovanillyl alcohol
Catalog No.:BCN7173
CAS No.:2380-78-1
- 4-Aminopyrazolo[3,4-d]pyrimidine
Catalog No.:BCC8690
CAS No.:2380-63-4
- Methylprednisolone Sodium Succinate
Catalog No.:BCC5629
CAS No.:2375-03-3
- Rivulobirin E
Catalog No.:BCN5090
CAS No.:237407-59-9
- 4-Amino-3-hydroxybenzoic acid
Catalog No.:BCC8681
CAS No.:2374-03-0
- trans-3'-O-Benzoyl-4'-O-methylkhellactone
Catalog No.:BCN6921
CAS No.:23733-95-1
- trans-Methylkhellactone
Catalog No.:BCN6919
CAS No.:23733-92-8
- 6-Hydroxy-4-Methylcoumarin
Catalog No.:BCC9206
CAS No.:2373-31-1
- Ambroxol HCl
Catalog No.:BCC5067
CAS No.:23828-92-4
- Liensinine perchlorate
Catalog No.:BCN6335
CAS No.:2385-63-9
- Ethyl2-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate
Catalog No.:BCC8979
CAS No.:238749-50-3
- Tosedostat (CHR2797)
Catalog No.:BCC2309
CAS No.:238750-77-1
- Zapotinin
Catalog No.:BCC9192
CAS No.:14813-20-8
- Boc-Lys(Z)-OH
Catalog No.:BCC2722
CAS No.:2389-45-9
- H-Lys(Boc)-OMe.HCl
Catalog No.:BCC2983
CAS No.:2389-48-2
- Z-Lys(Boc)-OH
Catalog No.:BCC2763
CAS No.:2389-60-8
- Dexamethasone dipropionate
Catalog No.:BCC8934
CAS No.:55541-30-5
- Alphaxalone
Catalog No.:BCC7545
CAS No.:23930-19-0
- 5-(2-Aminopropyl)-7-cyanoindolin-1-yl)propyl benzoate
Catalog No.:BCC8718
CAS No.:239463-72-0
- 5-[(2R)-2-Aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro-1H-indole-7-carbonitrile (2R,3R)-2,3-dihydroxybutanedioate
Catalog No.:BCN1479
CAS No.:239463-85-5
Steady-state luminescence investigation of the binding of Eu(III) and Tb(III) ions with synthetic peptides derived from plant thionins.[Pubmed:12161306]
J Inorg Biochem. 2002 Aug 15;91(2):363-70.
This work reports Eu(III) and Tb(III) luminescence titrations in which the lanthanide ions were used as spectroscopic probes for Ca(II) ions to determine the metal binding ability of Ac-NESVKEEGGW-NH(2) and Ac-NESVKEDGGW-NH(2). These decapeptides correspond to the putative calcium binding region of the plant antifungal proteins SI-alpha1 from Sorghum bicolor and of Zeathionin from Zea mays, respectively. The luminescence spectra for the Eu(III)-decapeptide system (red emission) with the excitation at the Trp band at 280 nm showed an enhancement of the intensities of the 5D(0)-->7F(J) transitions (where J=0-4) with increments of Eu(III) ion concentration. The photoluminescence titration data of the terbium ion (green emission) in the decapeptide solutions showed intensification of the 5D(4)-->7F(J) transitions (J=0-6), similar to that observed for the Eu(III) ion. Thus, energy transfer from Ac-NESVKEEGGW-NH(2) and Ac-NESVKEDGGW-NH(2) to the trivalent lanthanide ions revealed that these peptides are capable of binding to these metal ions with association constants of the order of 10(5) M(-1). The amino acid derivative Ac-Trp-OEt also transferred energy to Tb(III) and Eu(III) ions as judged from the quenching of tryptophan luminescence. However, the energy transfers were significantly lower. Taken together the luminescence titration data indicated that Ac-NESVKEEGGW-NH(2) and Ac-NESVKEDGGW-NH(2) bind efficiently to both trivalent lanthanide ions and that these ions may be used as probes to distinguish an anionic peptide from a neutral amino acid derivative.
Effect of thermodynamic water activity on amino-acid ester synthesis catalyzed by agarose-chymotrypsin in 3-pentanone.[Pubmed:1472540]
Biochim Biophys Acta. 1992 Dec 8;1156(1):67-70.
Chymotrypsin linked to agarose beads by multi-point covalent attachment catalyzes synthesis of Ac-Trp-OEt in 3-pentanone even when the thermodynamic water activity (aw) of the system is reduced to as low as 0.4. If fully hydrated catalyst is added to the reaction mixture before removal of water, product is formed linearly once aw has stabilized. The initial rate is reduced from that if aw is kept close to 1 (0.47 mmol s-1 (kg enzyme)-1), to 50% (aw 0.9), 25% (aw 0.4) and < 1% (aw 0.25). The large drop between aw of 1 and 0.9 probably reflects the effects of water removal on the agarose gel structure. Catalyst partly dried (even only to aw 0.86) before adding to the organic phase is inactive. At reduced aw, the equilibrium (when reached) is shifted in favor of the ester, as expected.
Analysis of proteases involved in phagocytic activity of macrophages through the use of various amino acid esters.[Pubmed:6355080]
J Biochem. 1983 Aug;94(2):565-73.
Inhibitory effects of various amino acid esters on the phagocytic activity of guinea pig peritoneal macrophages were studied with sensitized 51Cr-sheep erythrocytes (51Cr-EAb) as well as 125I-alpha-amylase complexed with homologous IgG2 antibody (Ag-Ab complex). The intracellular uptake of 51Cr-EAb was markedly inhibited by N-acetyl-L-phenylalanine ethyl ester (Ac-Phe-OEt), N-acetyl-L-tryptophan ethyl ester (Ac-Trp-OEt) and N-benzoyl-L-tyrosine ethyl ester (Bz-Tyr-OEt), but not by N-acetyl-L-tyrosine ethyl ester (Ac-Tyr-OEt), N-alpha-acetyl-L-arginine methyl ester (Ac-Arg-OMe), N-alpha-benzoyl-L-arginine ethyl ester (Bz-Arg-OEt) or N-alpha-acetyl-L-lysine methyl ester (Ac-Lys-OMe). When phagocytosis of the Ag-Ab complex was assayed by measuring the amount of digested products released from macrophage cells, Ac-Tyr-OEt also inhibited it as markedly as Ac-Phe-OEt, Ac-Trp-OEt, and Bz-Tyr-OEt did, whereas Bz-Arg-OEt again did not show any effect. The results of analysis of the intracellular fate of the Ag-Ab complex taken up by macrophages through the use of analytical density gradient fractionation of the homogenized cells suggest that Ac-Phe-OEt inhibits the ingestive process since the distribution of Ag-Ab complex showed a single peak, closely accompanying the plasma membrane. Ac-Tyr-OEt, on the other hand, caused a marked accumulation of Ag-Ab complex in the lysosome fraction, reflecting the inhibition of intralysosomal digestion of the complex.(ABSTRACT TRUNCATED AT 250 WORDS)
Circular dichroism and difference spectra of members of the troponin-tropomyosin system in cetyltrimethylammonium bromide.[Pubmed:1000361]
Can J Biochem. 1976 Nov;54(11):941-5.
The detergent cetyltrimethylammonium bromide (CTAB) was used as a perturbant to study protein structure. Low concentrations of CTAB induced difference spectra for Ac-Trp-OEt and Ac-Tyr-OEt. The delta epsilonM values at their difference maxima were found to be 1300 at 292 nm for Ac-Trp-OEt and 400 at 287 for Ac-Tyr-OEt. These values were used to determine the number of tyrosine residues exposed in tropomyosin and troponin C, as well as the tyrosine and tryptophan residues exposed in troponin I and troponin T. In tropomyosin and troponin C all of the tyorosine residues were accessible to detergent. For TN-T, three of four tyrosines were free while the tryptophan residues were only partially exposed. In the case of TN-I both tyrosines were fully exposed but again evidence was obtained for a partially buried tryptophan chromophore. The stability of these proteins to CTAB was studies by measuring the far-uv circular dichroism spectra. Tropomyosin was quite sensitive to detergent and suffered a 60% loss in ellipticity at the concentration of CTAB used. The troponins, on the other hand, were affected to a lesser extent.
Interaction between proteins and detergents which contain a hydrocarbon chain longer than 16 carbon atoms. II. Difference spectra of various proteins in cetyldimethyl-benzylammonium chloride.[Pubmed:237567]
Biochim Biophys Acta. 1975 May 30;393(1):215-24.
The detergents which contain a hydrocarbon side chain longer than 16 cabron atoms were used as a perturbant for the study of protein structure. ta low concentration of cetyldimethylbenzylammonium chloride (CDBA) caused difference spectra for Ac-Trp-OEt and AC-Tyr-OEt. The delta e values at their difference maxima became constant above 30 mM of cetyldimethylbenzylammonium chloride, 1430 at 294 nm for Ac-Trp-OEt and 450 at 288 nm for Ac-Tyr-OEt. These delta e values are higher than any other delta e values resulting from solvent effects by such a remarkably low concentration of organic reagents described in the literature so far. The absence of denaturation blue shift in the difference spectra and the fact that the optical rotatory dispersion of the proteins examined in the present study was not changed significantly by cetyldimethylbenzylammonium chloride indicate that the secondary and tertiary structures of the proteins were not destroyed by cetyldimethylbenzylammonium chloride. These characteristics, together with small overlapping of their difference spectra at 288 and 294 nm were advantageous in the determination of tryptophan and tyrosine residues exposed in glucagon, insulin and alcohol dehydrogenase from yeast. No tyrosine residues in ribonuclease A was accessible to cetyldimethylbenzylammonium chloride. Unusual difference spectrum with a peak at 298 nm was observed for lysozyme which is known to contain tryptophan residues in special environments. Ovalbumin gave a novel unusual difference spectrum with a peak at 290 nm and a shoulder at 298 nm, showing the existence of unusual tryptophan and probably tyrosine residues in the molecule.


