PodophyllotoxinAntineoplastic CAS# 518-28-5 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- 7-Xylosyltaxol
Catalog No.:BCN5341
CAS No.:90332-66-4
Quality Control & MSDS
Number of papers citing our products

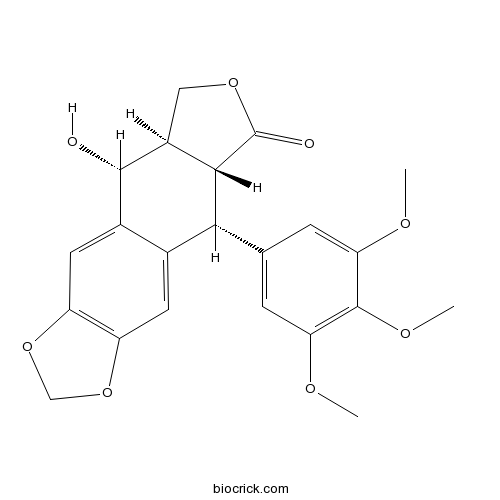
Chemical structure
3D structure
| Cas No. | 518-28-5 | SDF | Download SDF |
| PubChem ID | 10607 | Appearance | White powder |
| Formula | C22H22O8 | M.Wt | 414.41 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Synonyms | Podofilox; PPT;4354-76-1;Condyline;477-47-4 | ||
| Solubility | DMSO : ≥ 100 mg/mL (241.31 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (5R,5aR,8aR,9R)-5-hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one | ||
| SMILES | COC1=CC(=CC(=C1OC)OC)C2C3C(COC3=O)C(C4=CC5=C(C=C24)OCO5)O | ||
| Standard InChIKey | YJGVMLPVUAXIQN-XVVDYKMHSA-N | ||
| Standard InChI | InChI=1S/C22H22O8/c1-25-16-4-10(5-17(26-2)21(16)27-3)18-11-6-14-15(30-9-29-14)7-12(11)20(23)13-8-28-22(24)19(13)18/h4-7,13,18-20,23H,8-9H2,1-3H3/t13-,18+,19-,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Podophyllotoxin(Podofilox ) is a potent inhibitor of microtubule assembly and DNA topoisomerase II. Podophyllotoxin has antitumor and antiviral properties, but it also shows cytotoxicity for normal cells and hence side effects derived from its lack of selectivity against tumoral cells. |
| Targets | Topoisomerase | MMP(e.g.TIMP) |
| In vitro | Recent Developments Towards Podophyllotoxin Congeners as Potential Apoptosis Inducers.[Pubmed: 25469512]Anticancer Agents Med Chem. 2014 Nov 30.Podophyllotoxin, a lignan extracted from rhizomes of Podophyllum species, is a well established lead in the development of new chemical agents for the treatment of cancer. Its semi-synthetic variant, etoposide is an anticancer drug which inhibits DNA topoisomerase II causing cell cycle arrest in the S the phase. Its clinical success and intriguing mode of action made it a much sought after skeleton for the development of better antitumor agents. Modifications were made at several positions of its skeleton with the aim to either improve its potency or to overcome drug resistance. In recent years, the structurally modified Podophyllotoxins have been investigated for their apoptosis inducing ability. Although numerous reviews emphasized the occurrence, synthesis and applications of Podophyllotoxins, the recent progress towards development of structurally modified Podophyllotoxins possessing apoptosis inducing ability has not been previously reviewed. Apoptosis of human gastric cancer SGC-7901 cells induced by podophyllotoxin.[Pubmed: 24940431]Exp Ther Med. 2014 May;7(5):1317-1322. Epub 2014 Mar 6.Numerous studies have demonstrated that Podophyllotoxin and its derivatives exhibit antitumor effects. The aim of the present study was to investigate SGC-7901 cell apoptosis and the underlying mechanism induced by Podophyllotoxin. SGC-7901 cells were treated with varying concentrations of Podophyllotoxin. |
| Structure Identification | Curr Pharm Des. 2000 Dec;6(18):1811-39.Antitumor properties of podophyllotoxin and related compounds.[Pubmed: 11102564]The lignan family of natural products includes compounds with important antineoplastic and antiviral properties such as Podophyllotoxin and two of their semisynthetic derivatives, etoposide and teniposide. The latter are included in a wide variety of cancer chemotherapy protocols. Due to these biological activities, lignans, and especially cyclolignans, have been the objective of numerous studies focused to prepare better and safer anticancer drugs. The mechanism by which Podophyllotoxin blocks cell division is related to its inhibition of microtubule assembly in the mitotic apparatus. However, etoposide and teniposide were shown not to be inhibitors of microtubule assembly which suggested that their antitumor properties were due to another mechanism of action, via their interaction with DNA and inhibition of DNA topoisomerase II.
|

Podophyllotoxin Dilution Calculator

Podophyllotoxin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4131 mL | 12.0653 mL | 24.1307 mL | 48.2614 mL | 60.3267 mL |
| 5 mM | 0.4826 mL | 2.4131 mL | 4.8261 mL | 9.6523 mL | 12.0653 mL |
| 10 mM | 0.2413 mL | 1.2065 mL | 2.4131 mL | 4.8261 mL | 6.0327 mL |
| 50 mM | 0.0483 mL | 0.2413 mL | 0.4826 mL | 0.9652 mL | 1.2065 mL |
| 100 mM | 0.0241 mL | 0.1207 mL | 0.2413 mL | 0.4826 mL | 0.6033 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Podophyllotoxin, a kind of non-alkaloid toxin lignan extracted from the roots and rhizomes of Podophyllum plant, has been shown to inhibit the growth of various carcinoma cells. Podophyllotoxin is a natural product that inhibits the polymerization of tubulin and has served as a prototype for the development of diverse antitumor agents in clinical use.
- Evodiamine
Catalog No.:BCN1092
CAS No.:518-17-2
- Dehydroglyasperin D
Catalog No.:BCN6829
CAS No.:517885-72-2
- Rengynic acid
Catalog No.:BCN5644
CAS No.:517883-38-4
- Carteolol HCl
Catalog No.:BCC6466
CAS No.:51781-21-6
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
- Valechlorine
Catalog No.:BCN2763
CAS No.:51771-49-4
- Estra-4,9-diene-3,17-dione
Catalog No.:BCC8959
CAS No.:5173-46-6
- Uncarine E
Catalog No.:BCC8263
CAS No.:5171-37-9
- Shikonine
Catalog No.:BCN3530
CAS No.:517-89-5
- Shikonin
Catalog No.:BCN1006
CAS No.:517-88-4
- Dicentrine
Catalog No.:BCN3296
CAS No.:517-66-8
- Stephanine
Catalog No.:BCN5643
CAS No.:517-63-5
- (-)-beta-Peltatin
Catalog No.:BCN3606
CAS No.:518-29-6
- Tetrandrine
Catalog No.:BCN5955
CAS No.:518-34-3
- Corydaline
Catalog No.:BCN2342
CAS No.:518-69-4
- Emodin
Catalog No.:BCN5649
CAS No.:518-82-1
- Xanthopurpurin
Catalog No.:BCN6723
CAS No.:518-83-2
- Cycleanine
Catalog No.:BCN8445
CAS No.:518-94-5
- Isomaculosidine
Catalog No.:BCN7069
CAS No.:518-96-7
- 3,3'-Di-O-methylellagic acid 4'-glucoside
Catalog No.:BCN1431
CAS No.:51803-68-0
- Nimesulide
Catalog No.:BCC4435
CAS No.:51803-78-2
- Oxoepistephamiersine
Catalog No.:BCN5645
CAS No.:51804-68-3
- Dihydrooxoepistephamiersine
Catalog No.:BCN5646
CAS No.:51804-69-4
- Raltegravir (MK-0518)
Catalog No.:BCC2137
CAS No.:518048-05-0
Apoptosis of human gastric cancer SGC-7901 cells induced by podophyllotoxin.[Pubmed:24940431]
Exp Ther Med. 2014 May;7(5):1317-1322.
Numerous studies have demonstrated that Podophyllotoxin and its derivatives exhibit antitumor effects. The aim of the present study was to investigate SGC-7901 cell apoptosis and the underlying mechanism induced by Podophyllotoxin. SGC-7901 cells were treated with varying concentrations of Podophyllotoxin. MTT assays and flow cytometry were used to evaluate the effects of Podophyllotoxin on the proliferation and apoptosis of SGC-7901 cells, while fluorescence inverted microscopy was used to observe the morphology of SGC-7901 cells that had been dyed with Hoechst 33258. In addition, laser scanning confocal microscopy was used to analyze the mitochondrial membrane potential (MMP) of SGC-7901 cells dyed with Rhodamine 123. Western blotting was performed to analyze the expression levels of cytochrome c (cyt-c), caspase-9 and caspase-3 in the SGC-7901 cells. The results indicated that Podophyllotoxin was capable of inhibiting growth and inducing the apoptosis of SGC-7901 cells in a dose-dependent manner, causing cell cycle arrest at the G2/M phase. After 48 h of treatment, the apoptotic morphology of SGC-7901 cells was clear, exhibiting cell protuberance, concentrated cytoplasms and apoptotic bodies. Following 24 h of treatment, the MMP of the SGC-7901 cells decreased. In addition, after 48 h, the expression of cyt-c was shown to be upregulated, while the expression levels of pro-caspase-9 and pro-caspase-3 in the SGC-7901 cells were shown to be downregulated. In conclusion, apoptosis can be induced in SGC-7901 cells by Podophyllotoxin, potentially via a mitochondrial pathway, indicating that Podophyllotoxin may be a potent agent for cancer treatment.
Recent developments towards podophyllotoxin congeners as potential apoptosis inducers.[Pubmed:25469512]
Anticancer Agents Med Chem. 2015;15(5):565-74.
Podophyllotoxin, a lignan extracted from rhizomes of Podophyllum species, is a well established lead in the development of new chemical agents for the treatment of cancer. Its semi-synthetic variant, etoposide is an anticancer drug which inhibits DNA topoisomerase II causing cell cycle arrest in the S the phase. Its clinical success and intriguing mode of action made it a much sought after skeleton for the development of better antitumor agents. Modifications were made at several positions of its skeleton with the aim to either improve its potency or to overcome drug resistance. In recent years, the structurally modified Podophyllotoxins have been investigated for their apoptosis inducing ability. Although numerous reviews emphasized the occurrence, synthesis and applications of Podophyllotoxins, the recent progress towards development of structurally modified Podophyllotoxins possessing apoptosis inducing ability has not been previously reviewed. Therefore the present review focuses on the studies carried out in the design and synthesis of new Podophyllotoxin derivatives and their evaluation as apoptosis inducers.
Antitumor properties of podophyllotoxin and related compounds.[Pubmed:11102564]
Curr Pharm Des. 2000 Dec;6(18):1811-39.
The lignan family of natural products includes compounds with important antineoplastic and antiviral properties such as Podophyllotoxin and two of their semisynthetic derivatives, etoposide and teniposide. The latter are included in a wide variety of cancer chemotherapy protocols. Due to these biological activities, lignans, and especially cyclolignans, have been the objective of numerous studies focused to prepare better and safer anticancer drugs. The mechanism by which Podophyllotoxin blocks cell division is related to its inhibition of microtubule assembly in the mitotic apparatus. However, etoposide and teniposide were shown not to be inhibitors of microtubule assembly which suggested that their antitumor properties were due to another mechanism of action, via their interaction with DNA and inhibition of DNA topoisomerase II. Other Podophyllotoxin derivatives has also been reported which retained or even improved the cytotoxic activity, but these were weak inhibitors of topoisomerase II in vitro; the data revealed that such analogs exhibit a different, as yet unknown, mechanism of action. The main deficiency of these compounds is their cytotoxicity for normal cells and hence side effects derived from their lack of selectivity against tumoral cells. In this regard it is necessary to investigate and prepare new more potent and less toxic analogs, that is, with better therapeutic indices. It is well accepted from structure-activity studies in this field that the trans-lactones are more potent as antineoplastics than the cis-lactones. Not only the configuration of the D ring is an important factor for high cytotoxic activity, but also a quasi-axial arrangement of the E ring is necessary. On this basis, studies on lignans have been addressed to modify the lactone moiety and prepare analogs with heteroatoms at different positions of the cyclolignan skeleton. Our group has been working during the last few years on chemical transformations of Podophyllotoxin and analogs and we have prepared a large number of cyclolignan derivatives some of which display potent antiviral, immunosuppressive and cytotoxic activities. We have reported several new cytotoxic agents with nitrogen atoms at C-7 or C-9 or at both C-7 and C-9: imine derivatives, oxime derivatives, pyrazoline-, pyrazo- and isoxazoline-fused cyclolignans. At present, we are preparing mainly new compounds by modifications of the A and E cyclolignan-rings. They are being tested on cultures of different tumoral cell lines (P-388 murine leukemia, A-549 human lung carcinoma, HT-29 human colon carcinoma and MEL-28 human melanoma) and some of them have shown an interesting and selective cytotoxicity.


