187-1, N-WASP inhibitorN-WASP inhibitor; inhibits actin assembly CAS# 380488-27-7 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- MRK 560
Catalog No.:BCC2345
CAS No.:677772-84-8
- PF-03084014
Catalog No.:BCC1848
CAS No.:865773-15-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 380488-27-7 | SDF | Download SDF |
| PubChem ID | 44405676 | Appearance | Powder |
| Formula | C96H122N18O16 | M.Wt | 1784.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in water | ||
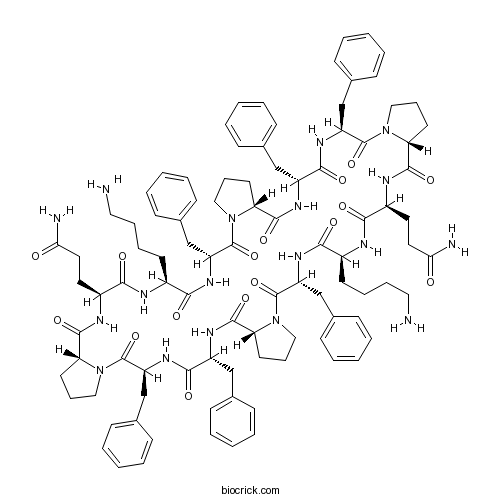
| Sequence | KFPFFPQ KFPFFPQ (Modifications: Phe-2 = D-Phe, Pro-3 = D-Pro, Phe-4 = D-Phe, Pro-6 = D-Pro, Cyclized) | ||
| SMILES | C1CC2C(=O)NC(C(=O)NC(C(=O)N3CCCC3C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N4CCCC4C(=O)NC(C(=O)NC(C(=O)N5CCCC5C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N2C1)CC6=CC=CC=C6)CCCCN)CCC(=O)N)CC7=CC=CC=C7)CC8=CC=CC=C8)CC9=CC=CC=C9)CCCCN)CCC(=O)N)CC1=CC=CC=C1)CC1=CC=CC=C1 | ||
| Standard InChIKey | PHTIQAVQWWOKNF-RNEDXEELSA-N | ||
| Standard InChI | InChI=1S/C96H122N18O16/c97-49-21-19-39-67-83(117)107-73(57-63-31-11-3-12-32-63)93(127)113-53-25-43-79(113)91(125)105-71(55-61-27-7-1-8-28-61)87(121)109-75(59-65-35-15-5-16-36-65)95(129)111-51-23-41-77(111)89(123)103-69(45-47-81(99)115)85(119)102-68(40-20-22-50-98)84(118)108-74(58-64-33-13-4-14-34-64)94(128)114-54-26-44-80(114)92(126)106-72(56-62-29-9-2-10-30-62)88(122)110-76(60-66-37-17-6-18-38-66)96(130)112-52-24-42-78(112)90(124)104-70(86(120)101-67)46-48-82(100)116/h1-18,27-38,67-80H,19-26,39-60,97-98H2,(H2,99,115)(H2,100,116)(H,101,120)(H,102,119)(H,103,123)(H,104,124)(H,105,125)(H,106,126)(H,107,117)(H,108,118)(H,109,121)(H,110,122)/t67-,68-,69-,70-,71+,72+,73+,74+,75-,76-,77+,78+,79+,80+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibits neural Wiskott-Aldrich syndrome protein (N-WASP) by stabilizing the autoinhibited state of the protein. Blocks phosphatidylinositol 4,5-bisphosphate (PIP2)-stimulated actin assembly (IC50 ~ 2 μM) but does not directly inhibit actin polymerization. |

187-1, N-WASP inhibitor Dilution Calculator

187-1, N-WASP inhibitor Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- alpha-Epoxydihydroartemisinic acid
Catalog No.:BCN5434
CAS No.:380487-65-0
- NBMPR
Catalog No.:BCC7516
CAS No.:38048-32-7
- (+)-columbianetin
Catalog No.:BCN2331
CAS No.:3804-70-4
- Tenovin-1
Catalog No.:BCC2239
CAS No.:380315-80-0
- Sulfamonomethoxine sodium
Catalog No.:BCC9157
CAS No.:38006-08-5
- 2-Amino-4'-fluorobenzophenone
Catalog No.:BCC8530
CAS No.:3800-06-4
- LM 22A4
Catalog No.:BCC6239
CAS No.:37988-18-4
- Geranylacetone
Catalog No.:BCN7567
CAS No.:3796-70-1
- tenofovir diphosphate
Catalog No.:BCC6447
CAS No.:166403-66-3
- Tenofovir Alafenamide Fumarate
Catalog No.:BCC8067
CAS No.:379270-38-9
- Tenofovir alafenamide
Catalog No.:BCC8066
CAS No.:379270-37-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- DQP 1105
Catalog No.:BCC6205
CAS No.:380560-89-4
- CMPDA
Catalog No.:BCC6151
CAS No.:380607-77-2
- [(pF)Phe4]Nociceptin(1-13)NH2
Catalog No.:BCC5778
CAS No.:380620-88-2
- Pterolactam
Catalog No.:BCN5435
CAS No.:38072-88-7
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- TACA
Catalog No.:BCC6564
CAS No.:38090-53-8
- Perampanel
Catalog No.:BCC1847
CAS No.:380917-97-5
- Streptomycin sulfate
Catalog No.:BCC4851
CAS No.:3810-74-0
- Brevianamide F
Catalog No.:BCN6452
CAS No.:38136-70-8
- 28-Hydroxy-3-oxoolean-12-en-29-oic acid
Catalog No.:BCN1449
CAS No.:381691-22-1
- Bephenium Hydroxynaphthoate
Catalog No.:BCC3735
CAS No.:3818-50-6
- 7,8-Dihydroxyflavone
Catalog No.:BCC6072
CAS No.:38183-03-8
A chemical inhibitor of N-WASP reveals a new mechanism for targeting protein interactions.[Pubmed:11553809]
Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10624-9.
Cell morphology and motility are governed largely by complex signaling networks that ultimately engage the actin cytoskeleton. Understanding how individual circuits contribute to the process of forming cellular structures would be aided greatly by the availability of specific chemical inhibitors. We have used a novel chemical screen in Xenopus cell-free extracts to identify compounds that inhibit signaling pathways regulating actin polymerization. Here we report the results of a high-throughput screen for compounds that inhibit phosphatidylinositol 4,5-bisphosphate (PIP(2))-induced actin assembly and the identification of the first compound, a cyclic peptide, known to block actin assembly by inhibiting an upstream signaling component. We identify the target of this compound as N-WASP, a protein that has been investigated for its role as a node interconnecting various actin signaling networks. We show that this compound prevents activation of the Arp2/3 complex by N-WASP by allosterically stabilizing the autoinhibited conformation of N-WASP.
N-WASP inhibitor wiskostatin nonselectively perturbs membrane transport by decreasing cellular ATP levels.[Pubmed:17092993]
Am J Physiol Cell Physiol. 2007 Apr;292(4):C1562-6.
Wiskott-Aldrich syndrome protein (WASP) and WAVE stimulate actin-related protein (Arp)2/3-mediated actin polymerization, leading to diverse downstream effects, including the formation and remodeling of cell surface protrusions, modulation of cell migration, and intracytoplasmic propulsion of organelles and pathogens. Selective inhibitors of individual Arp2/3 activators would enable more exact dissection of WASP- and WAVE-dependent cellular pathways and are potential therapeutic targets for viral pathogenesis. Wiskostatin is a recently described chemical inhibitor that selectively inhibits neuronal WASP (N-WASP)-mediated actin polymerization in vitro. A growing number of recent studies have utilized this drug in vivo to uncover novel cellular functions for N-WASP; however, the selectivity of wiskostatin in intact cells has not been carefully explored. In our studies with this drug, we observed rapid and dose-dependent inhibition of N-WASP-dependent membrane trafficking steps. Additionally, however, we found that addition of wiskostatin inhibited numerous other cellular functions that are not believed to be N-WASP dependent. Further studies revealed that wiskostatin treatment caused a rapid, profound, and irreversible decrease in cellular ATP levels, consistent with its global effects on cell function. Our data caution against the use of this drug as a selective perturbant of N-WASP-dependent actin dynamics in vivo.
How signaling proteins integrate multiple inputs: a comparison of N-WASP and Cdk2.[Pubmed:11891112]
Curr Opin Cell Biol. 2002 Apr;14(2):149-54.
Signal transduction proteins that can integrate multiple upstream signals play a critical role in the complex regulatory circuits that control cellular behavior. The two signaling node proteins cyclin-dependent kinase 2 and the actin regulator neuronal Wiskott-Aldrich syndrome protein have qualitatively similar signaling properties. Recent studies, however, reveal that these proteins utilize distinct mechanisms of signal integration, leading to subtle but important quantitative differences in behavior.
Identification of another actin-related protein (Arp) 2/3 complex binding site in neural Wiskott-Aldrich syndrome protein (N-WASP) that complements actin polymerization induced by the Arp2/3 complex activating (VCA) domain of N-WASP.[Pubmed:11432863]
J Biol Chem. 2001 Aug 31;276(35):33175-80.
Neural Wiskott-Aldrich syndrome protein (N-WASP) is an essential regulator of actin cytoskeleton formation via its association with the actin-related protein (Arp) 2/3 complex. It is believed that the C-terminal Arp2/3 complex-activating domain (verprolin homology, cofilin homology, and acidic (VCA) or C-terminal region of WASP family proteins domain) of N-WASP is usually kept masked (autoinhibition) but is opened upon cooperative binding of upstream regulators such as Cdc42 and phosphatidylinositol 4,5-bisphosphate (PIP2). However, the mechanisms of autoinhibition and association with Arp2/3 complex are still unclear. We focused on the acidic region of N-WASP because it is thought to interact with Arp2/3 complex and may be involved in autoinhibition. Partial deletion of acidic residues from the VCA portion alone greatly reduced actin polymerization activity, demonstrating that the acidic region contributes to Arp2/3 complex-mediated actin polymerization. Surprisingly, the same partial deletion of the acidic region in full-length N-WASP led to constitutive activity comparable with the activity seen with the VCA portion. Therefore, the acidic region in full-length N-WASP plays an indispensable role in the formation of the autoinhibited structure. This mutant contains WASP-homology (WH) 1 domain with weak affinity to the Arp2/3 complex, leading to activity in the absence of part of the acidic region. Furthermore, the actin comet formed by the DeltaWH1 mutant of N-WASP was much smaller than that of wild-type N-WASP. Partial deletion of acidic residues did not affect actin comet size, indicating the importance of the WH1 domain in actin structure formation. Collectively, the acidic region of N-WASP plays an essential role in Arp2/3 complex activation as well as in the formation of the autoinhibited structure, whereas the WH1 domain complements the activation of the Arp2/3 complex achieved through the VCA portion.


