Tenofovir alafenamideCAS# 379270-37-8 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 379270-37-8 | SDF | Download SDF |
| PubChem ID | 9574768 | Appearance | Powder |
| Formula | C21H29N6O5P | M.Wt | 476.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GS-7340; GS 7340 | ||
| Solubility | DMSO : ≥ 31 mg/mL (65.06 mM) H2O : 6.67 mg/mL (14.00 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
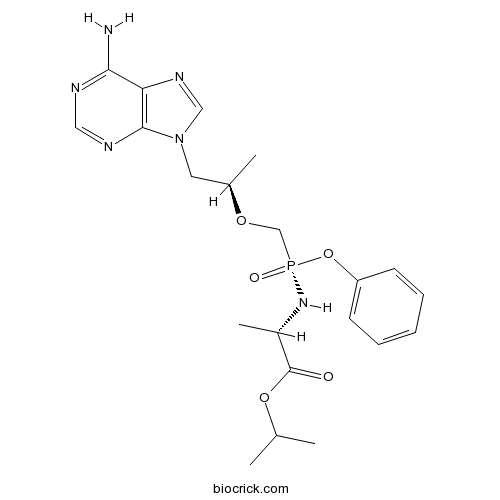
| Chemical Name | propan-2-yl (2S)-2-[[[(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethyl-phenoxyphosphoryl]amino]propanoate | ||
| SMILES | CC(C)OC(=O)C(C)NP(=O)(COC(C)CN1C=NC2=C1N=CN=C2N)OC3=CC=CC=C3 | ||
| Standard InChIKey | LDEKQSIMHVQZJK-CAQYMETFSA-N | ||
| Standard InChI | InChI=1S/C21H29N6O5P/c1-14(2)31-21(28)16(4)26-33(29,32-17-8-6-5-7-9-17)13-30-15(3)10-27-12-25-18-19(22)23-11-24-20(18)27/h5-9,11-12,14-16H,10,13H2,1-4H3,(H,26,29)(H2,22,23,24)/t15-,16+,33+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GS-7340(Tenofovir alafenamide) is a prodrug of tenofovir (TFV) that more efficiently delivers TFV into lymphoid cells and tissues than TFV disoproxil fumarate.
IC50 value:
Target: NRTI; HIV reverse transcriptase inhibitor
GS-7340 reduces first-pass clearance to be an effective oral prodrug, its permeability and stability were characterized in vitro and detailed pharmacokinetic studies were completed in dogs. GS-7340 showed concentration-dependent permeability through monolayers of caco-2 cells and dose-dependent oral bioavailability in dogs, increasing from 1.7% at 2 mg/kg to 24.7% at 20 mg/kg, suggesting saturable intestinal efflux transport [1]. Significant reductions in plasma HIV-1 RNA from baseline to day 11 were observed for all TAF dose groups compared with placebo (P < 0.01), with a median decrease of 1.08-1.73 log10 copies per milliliter, including a dose-response relationship for viral load decrease up to 25 mg [2]. References: | |||||

Tenofovir alafenamide Dilution Calculator

Tenofovir alafenamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0988 mL | 10.4938 mL | 20.9877 mL | 41.9754 mL | 52.4692 mL |
| 5 mM | 0.4198 mL | 2.0988 mL | 4.1975 mL | 8.3951 mL | 10.4938 mL |
| 10 mM | 0.2099 mL | 1.0494 mL | 2.0988 mL | 4.1975 mL | 5.2469 mL |
| 50 mM | 0.042 mL | 0.2099 mL | 0.4198 mL | 0.8395 mL | 1.0494 mL |
| 100 mM | 0.021 mL | 0.1049 mL | 0.2099 mL | 0.4198 mL | 0.5247 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GS-7340(Tenofovir alafenamide) is a prodrug of tenofovir (TFV) that more efficiently delivers TFV into lymphoid cells and tissues than TFV disoproxil fumarate.
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Cimifugin
Catalog No.:BCN5433
CAS No.:37921-38-3
- Jolkinolide B
Catalog No.:BCN2391
CAS No.:37905-08-1
- Jolkinolide A
Catalog No.:BCN3771
CAS No.:37905-07-0
- Scarlet 808
Catalog No.:BCC9139
CAS No.:3789-75-1
- Ro 08-2750
Catalog No.:BCC7307
CAS No.:37854-59-4
- Nepodin
Catalog No.:BCN6894
CAS No.:3785-24-8
- Germacrene D
Catalog No.:BCN3851
CAS No.:37839-63-7
- Phaseollidin
Catalog No.:BCN5432
CAS No.:37831-70-2
- ACDPP hydrochloride
Catalog No.:BCC7302
CAS No.:37804-11-8
- Betamethasone
Catalog No.:BCC4765
CAS No.:378-44-9
- Cimigenol
Catalog No.:BCC8149
CAS No.:3779-59-7
- Tenofovir Alafenamide Fumarate
Catalog No.:BCC8067
CAS No.:379270-38-9
- tenofovir diphosphate
Catalog No.:BCC6447
CAS No.:166403-66-3
- Geranylacetone
Catalog No.:BCN7567
CAS No.:3796-70-1
- LM 22A4
Catalog No.:BCC6239
CAS No.:37988-18-4
- 2-Amino-4'-fluorobenzophenone
Catalog No.:BCC8530
CAS No.:3800-06-4
- Sulfamonomethoxine sodium
Catalog No.:BCC9157
CAS No.:38006-08-5
- Tenovin-1
Catalog No.:BCC2239
CAS No.:380315-80-0
- (+)-columbianetin
Catalog No.:BCN2331
CAS No.:3804-70-4
- NBMPR
Catalog No.:BCC7516
CAS No.:38048-32-7
- alpha-Epoxydihydroartemisinic acid
Catalog No.:BCN5434
CAS No.:380487-65-0
- 187-1, N-WASP inhibitor
Catalog No.:BCC5866
CAS No.:380488-27-7
- DQP 1105
Catalog No.:BCC6205
CAS No.:380560-89-4
Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis.[Pubmed:25896688]
Antimicrob Agents Chemother. 2015 Jul;59(7):3913-9.
Oral or topical daily administration of antiretroviral (ARV) drugs to HIV-1-negative individuals in vulnerable populations is a promising strategy for HIV-1 prevention. Adherence to the dosing regimen has emerged as a critical factor determining efficacy outcomes of clinical trials. Because adherence to therapy is inversely related to the dosing period, sustained release or long-acting ARV formulations hold significant promise for increasing the effectiveness of HIV-1 preexposure prophylaxis (PrEP) by reducing dosing frequency. A novel, subdermal implant delivering the potent prodrug Tenofovir alafenamide (TAF) with controlled, sustained, zero-order (linear) release characteristics is described. A candidate device delivering TAF at 0.92 mg day(-1) in vitro was evaluated in beagle dogs over 40 days for pharmacokinetics and preliminary safety. No adverse events related to treatment with the test article were noted during the course of the study, and no significant, unusual abnormalities were observed. The implant maintained a low systemic exposure to TAF (median, 0.85 ng ml(-1); interquartile range [IQR], 0.60 to 1.50 ng ml(-1)) and tenofovir (TFV; median, 15.0 ng ml(-1); IQR, 8.8 to 23.3 ng ml(-1)), the product of in vivo TAF hydrolysis. High concentrations (median, 512 fmol/10(6) cells over the first 35 days) of the pharmacologically active metabolite, TFV diphosphate, were observed in peripheral blood mononuclear cells at levels over 30 times higher than those associated with HIV-1 PrEP efficacy in humans. Our report on the first sustained-release nucleoside reverse transcriptase inhibitor (NRTI) for systemic delivery demonstrates a successful proof of principle and holds significant promise as a candidate for HIV-1 prophylaxis in vulnerable populations.
Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340.[Pubmed:22738467]
Mol Pharm. 2013 Feb 4;10(2):459-66.
GS-7340 is a prodrug of tenofovir (TFV) that more efficiently delivers TFV into lymphoid cells and tissues than the clinically used prodrug TFV disoproxil fumarate, resulting in higher antiviral potency at greatly reduced doses and lower systemic TFV exposure. First-pass extraction by the intestine and liver represents substantial barriers to the oral delivery of prodrugs designed for rapid intracellular hydrolysis. In order to understand how GS-7340 reduces first-pass clearance to be an effective oral prodrug, its permeability and stability were characterized in vitro and detailed pharmacokinetic studies were completed in dogs. GS-7340 showed concentration-dependent permeability through monolayers of caco-2 cells and dose-dependent oral bioavailability in dogs, increasing from 1.7% at 2 mg/kg to 24.7% at 20 mg/kg, suggesting saturable intestinal efflux transport. Taking into account a 65% hepatic extraction measured in portal vein cannulated dogs, high dose GS-7340 is nearly completely absorbed. Consistent with the proposed role of intestinal efflux transport, coadministration of low dose GS-7340 with a transport inhibitor substantially increased GS-7340 exposure. The result of effective oral absorption and efficient lymphoid cell loading was reflected in the high and persistent levels of the pharmacologically active metabolite, TFV diphosphate, in peripheral blood mononuclear cells following oral administration to dogs. In conclusion, GS-7340 reaches the systemic circulation to effectively load target cells by saturating intestinal efflux transporters, facilitated by its high solubility, and by maintaining sufficient stability in intestinal and hepatic tissue.
Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy.[Pubmed:25870059]
Antimicrob Agents Chemother. 2015;59(6):3563-9.
Tenofovir alafenamide (TAF) is a prodrug of tenofovir (TFV) currently in clinical evaluation for treatment for HIV and hepatitis B virus (HBV) infections. Since the target tissue for HBV is the liver, the hepatic delivery and metabolism of TAF in primary human hepatocytes in vitro and in dogs in vivo were evaluated here. Incubation of primary human hepatocytes with TAF resulted in high levels of the pharmacologically active metabolite tenofovir diphosphate (TFV-DP), which persisted in the cell with a half-life of >24 h. In addition to passive permeability, studies of transfected cell lines suggest that the hepatic uptake of TAF is also facilitated by the organic anion-transporting polypeptides 1B1 and 1B3 (OATP1B1 and OATP1B3, respectively). In order to inhibit HBV reverse transcriptase, TAF must be converted to the pharmacologically active form, TFV-DP. While cathepsin A is known to be the major enzyme hydrolyzing TAF in cells targeted by HIV, including lymphocytes and macrophages, TAF was primarily hydrolyzed by carboxylesterase 1 (CES1) in primary human hepatocytes, with cathepsin A making a small contribution. Following oral administration of TAF to dogs for 7 days, TAF was rapidly absorbed. The appearance of the major metabolite TFV in plasma was accompanied by a rapid decline in circulating TAF. Consistent with the in vitro data, high and persistent levels of TFV-DP were observed in dog livers. Notably, higher liver TFV-DP levels were observed after administration of TAF than those given TDF. These results support the clinical testing of once-daily low-dose TAF for the treatment of HBV infection.
Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro.[Pubmed:22850510]
Antimicrob Agents Chemother. 2012 Oct;56(10):5409-13.
The experimental pharmacoenhancer cobicistat (COBI), a potent mechanism-based inhibitor of cytochrome P450 3A enzymes, was found to inhibit the intestinal efflux transporters P-glycoprotein and breast cancer resistance protein. Consistent with its transporter inhibition, COBI significantly increased the absorptive flux of potential candidates for clinical coadministration, including the HIV protease inhibitors atazanavir and darunavir and the lymphoid cell- and tissue-targeted prodrug of the nucleotide analog tenofovir, GS-7340, through monolayers of Caco-2 cells in vitro.


