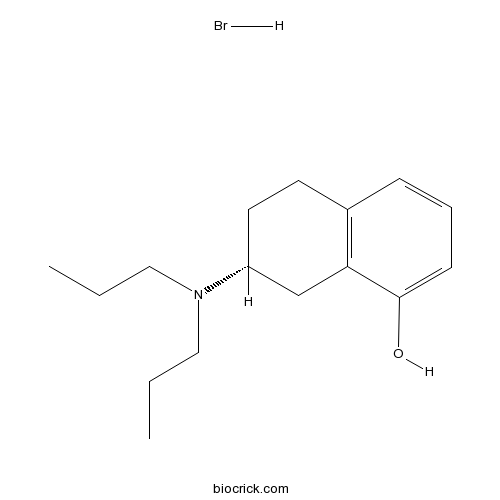
(R)-(+)-8-Hydroxy-DPAT hydrobromideCAS# 78095-19-9 |
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 78095-19-9 | SDF | Download SDF |
| PubChem ID | 11957570 | Appearance | Powder |
| Formula | C16H26BrNO | M.Wt | 328.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 75 mM in water and to 75 mM in DMSO | ||
| Chemical Name | (7R)-7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-ol;hydrobromide | ||
| SMILES | CCCN(CCC)C1CCC2=C(C1)C(=CC=C2)O.Br | ||
| Standard InChIKey | BATPBOZTBNNDLN-PFEQFJNWSA-N | ||
| Standard InChI | InChI=1S/C16H25NO.BrH/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14;/h5-7,14,18H,3-4,8-12H2,1-2H3;1H/t14-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Full 5-HT1A serotonin receptor agonist; more active enantiomer. Reduces hippocampal 5-HT levels following systemic administration in rats in vivo. More active enantiomer of 8-Hydroxy-DPAT hydrobromide. 7-Hydroxy-DPAT hydrobromide also available. |

(R)-(+)-8-Hydroxy-DPAT hydrobromide Dilution Calculator

(R)-(+)-8-Hydroxy-DPAT hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0461 mL | 15.2304 mL | 30.4609 mL | 60.9217 mL | 76.1522 mL |
| 5 mM | 0.6092 mL | 3.0461 mL | 6.0922 mL | 12.1843 mL | 15.2304 mL |
| 10 mM | 0.3046 mL | 1.523 mL | 3.0461 mL | 6.0922 mL | 7.6152 mL |
| 50 mM | 0.0609 mL | 0.3046 mL | 0.6092 mL | 1.2184 mL | 1.523 mL |
| 100 mM | 0.0305 mL | 0.1523 mL | 0.3046 mL | 0.6092 mL | 0.7615 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fmoc-Lys(Fmoc)-OH
Catalog No.:BCC3521
CAS No.:78081-87-5
- MMPX
Catalog No.:BCC6692
CAS No.:78033-08-6
- 1,3-Diacetylvilasinin
Catalog No.:BCN4580
CAS No.:78012-28-9
- Zeylasterone
Catalog No.:BCN8057
CAS No.:78012-25-6
- Linalool
Catalog No.:BCN6339
CAS No.:78-70-6
- Isophorone
Catalog No.:BCN8329
CAS No.:78-59-1
- Mulberrofuran C
Catalog No.:BCN4032
CAS No.:77996-04-4
- Secologanin dimethyl acetal
Catalog No.:BCN4581
CAS No.:77988-07-9
- Z-Glycinol
Catalog No.:BCC3095
CAS No.:77987-49-6
- Longikaurin E
Catalog No.:BCN4329
CAS No.:77949-42-9
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
- Aztreonam
Catalog No.:BCC2557
CAS No.:78110-38-0
- Okadaic acid
Catalog No.:BCC2464
CAS No.:78111-17-8
- DAMGO
Catalog No.:BCC6958
CAS No.:78123-71-4
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- CDPPB
Catalog No.:BCC7610
CAS No.:781652-57-1
- 4-Hydroxyisoleucine
Catalog No.:BCN1211
CAS No.:781658-23-9
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
- Nirtetralin
Catalog No.:BCN3755
CAS No.:78185-63-4
- 20(S)-Ginsenoside Rh2
Catalog No.:BCN1070
CAS No.:78214-33-2
- Paroxetine HCl
Catalog No.:BCC5054
CAS No.:78246-49-8
- [Orn5]-URP
Catalog No.:BCC5985
CAS No.:782485-03-4
5-HT1A Receptor Function Makes Wound Healing a Happier Process.[Pubmed:30618734]
Front Pharmacol. 2018 Dec 11;9:1406.
Skin wound healing is a multistage phenomenon that is regulated by cell-cell interplay and various factors. Endogenous serotonin is an important neurotransmitter and cytokine. Its interaction with the serotonin 1A receptor (5-HTR1A) delivers downstream cellular effects. The role of serotonin (5-hydroxytryptamine, 5-HT) and the 5-HT1A receptor has been established in the regeneration of tissues such as the liver and spinal motor neurons, prompting the investigation of the role of 5-HT1A receptor in skin healing. This study assessed the role of 5-HT1A receptor in excisional wound healing by employing an excisional punch biopsy model on 5-Ht1a receptor knockout mice. Post-harvest analysis revealed 5-Ht1a receptor knockout mice showed impaired skin healing, accompanied by a greater number of F4/80 macrophages, which prolongs the inflammatory phase of wound healing. To further unravel this phenomenon, we employed the 5-HT1A receptor agonist [(R)-(+)-8-Hydroxy-DPAT hydrobromide] as a topical cream treatment in an excisional punch biopsy model. The 5-HT1A receptor agonist treated group showed a smaller wound area, scar size, and improved neovascularization, which contributed to improve healing outcomes as compared to the control. Collectively, these findings revealed that serotonin and 5-HT1A receptor play an important role during the healing process. These findings may open new lines of investigation for the potential treatment alternatives to improve skin healing with minimal scarring.
Differential effects of (R)-, (R, S)- and (S)-8-hydroxy-2-(di-n-propylamino)tetralin on hippocampal serotonin release and induction of hypothermia in awake rats.[Pubmed:15050424]
Life Sci. 2004 Apr 23;74(23):2865-75.
The effects of (R)- and (S)-optical isomers of 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT) and of the racemate (R,S)-8-OH-DPAT on serotonin (5-HT) release in the ventral hippocampus of awake rats and on induction of the whole-body hypothermia were studied. Extracellular 5-HT levels were determined by a newly developed high-sensitive HPLC method based on derivatization with benzylamine and fluorescence detection. The basal levels of 5-HT in 20 min microdialysates from rats perfused with Ringer solution or with Ringer solution containing 1 microM citalopram were 6.3 +/- 1.3 fmol/20 microl and 36.1 +/- 4.2 fmol/20 microl (n=20), respectively. The reduction of hippocampal 5-HT levels induced by subcutaneous (s.c.) administration of (R,S)-8-OH-DPAT (0.3 mg/kg) was significantly attenuated by the presence of 5-HT reuptake inhibitor citalopram in Ringer solution only at its peak value at 40 min (maximal reduction to 60% compared to 46% of control values in Ringer-perfused rats), whereas the overall effects were comparable at both experimental conditions. Injection of (R)-8-OH-DPAT (0.3 mg/kg s.c.) caused further reduction of 5-HT levels, to 49% and 41%, respectively, whereas (S)-8-OH-DPAT (0.3 mg/kg s.c.) caused maximal reduction of 5-HT levels only to 74% of controls in both perfusion groups. Similar pattern and time-courses were observed in rats with hypothermia induced by injection of 8-OH-DPAT enantiomers, where (R,S), (R)-forms were about two-times more potent than the (S)-isomer. It is concluded that the acute systemic dose of (R)-, (S)- and (R,S)-8-OH-DPAT enantiomers exerted enantiomer-specific effects on 5-HT(1A) receptor-mediated function both at the presynaptic and postsynaptic sites as revealed by monitoring hippocampal 5-HT levels and body temperature.
Intrinsic activity of enantiomers of 8-hydroxy-2-(di-n-propylamino)tetralin and its analogs at 5-hydroxytryptamine1A receptors that are negatively coupled to adenylate cyclase.[Pubmed:1828859]
Mol Pharmacol. 1991 Jun;39(6):780-7.
Although many different types of compounds have been tested for 5-hydroxytryptamine1A (5-HT1A) binding affinity, much remains to be learned about the structural requirements associated with 5-HT1A agonism, partial agonism, and antagonism. The present study uses the forskolin-stimulated adenylate cyclase (FSC) assay as a functional screen in rat hippocampal membranes to examine structure-activity relationships for a series of enantiomers of novel analogs of the prototypic 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). The findings illustrate that there can be large enantiomeric differences in intrinsic activity at the 5-HT1A receptor, independent of enantiomeric effects on binding affinity. Generally, for each enantiomeric pair exhibiting stereoselective 5-HT1A binding, the enantiomer with the higher affinity also displayed the greater amount of 5-HT1A intrinsic activity in the FSC assay. Interestingly, the enantiomers of 8-OH-DPAT itself displayed stereoselective differences in intrinsic activity but not 5-HT1A affinity. Several of the compounds, namely (S)-UH-301, (2R,3R)-CM-12, and (1S,2R)-LEA-146, may have potential as prototypes for selective 5-HT1A antagonists, and (S)-UH-301 itself may be useful as a selective 5-HT1A antagonist. The FSC data presented here are in good agreement with reported measures of in vivo 5-HT1A activity, which were in part the basis of a recently proposed model for the 5-HT1A pharmacophore [J. Med. Chem. 34: 497-510 (1991)].
Resolved N,N-dialkylated 2-amino-8-hydroxytetralins: stereoselective interactions with 5-HT1A receptors in the brain.[Pubmed:2522991]
J Med Chem. 1989 Apr;32(4):779-83.
The enantiomers of the N,N-dimethylamino (1), N,N-diethylamino (2), and N,N-dibutylamino (4) derivatives of 8-hydroxy-2-(dipropylamino)tetralin (8-OH DPAT; 3) have been synthesized. The compounds have been tested for activity at central 5-hydroxytryptamine and dopamine receptors by use of biochemical and behavioral tests in rats. In addition, the ability of the enantiomers of 1-4 to displace [3H]-8-OH DPAT from 5-HT1A binding sites was evaluated. Rank order of potencies in the in vivo tests corresponded to that observed in the 5-HT1A binding assay. In all three tests, the enantiomeric potency ratio was about 10 for 1 and 2 and only around 2-4 for 3 and 4. The more potent enantiomer of 1-3 had the R configuration. In contrast, (S)-4 seemed to be slightly more potent than (R)-4.


