IsophoroneCAS# 78-59-1 |
Quality Control & MSDS
Number of papers citing our products

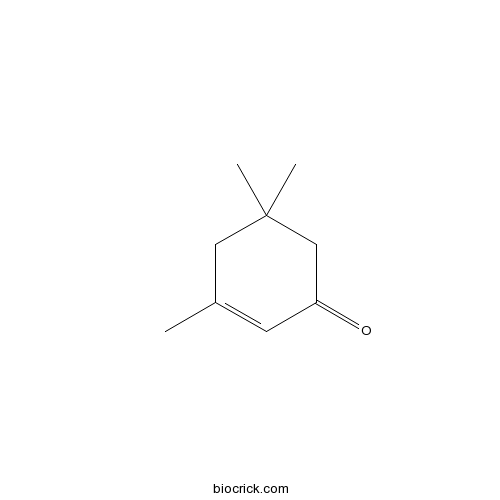
Chemical structure

3D structure
| Cas No. | 78-59-1 | SDF | Download SDF |
| PubChem ID | 6544 | Appearance | Colorless liquid |
| Formula | C9H14O | M.Wt | 138.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,5,5-trimethylcyclohex-2-en-1-one | ||
| SMILES | CC1=CC(=O)CC(C1)(C)C | ||
| Standard InChIKey | HJOVHMDZYOCNQW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H14O/c1-7-4-8(10)6-9(2,3)5-7/h4H,5-6H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isophorone Dilution Calculator

Isophorone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.2354 mL | 36.1768 mL | 72.3537 mL | 144.7073 mL | 180.8842 mL |
| 5 mM | 1.4471 mL | 7.2354 mL | 14.4707 mL | 28.9415 mL | 36.1768 mL |
| 10 mM | 0.7235 mL | 3.6177 mL | 7.2354 mL | 14.4707 mL | 18.0884 mL |
| 50 mM | 0.1447 mL | 0.7235 mL | 1.4471 mL | 2.8941 mL | 3.6177 mL |
| 100 mM | 0.0724 mL | 0.3618 mL | 0.7235 mL | 1.4471 mL | 1.8088 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mulberrofuran C
Catalog No.:BCN4032
CAS No.:77996-04-4
- Secologanin dimethyl acetal
Catalog No.:BCN4581
CAS No.:77988-07-9
- Z-Glycinol
Catalog No.:BCC3095
CAS No.:77987-49-6
- Longikaurin E
Catalog No.:BCN4329
CAS No.:77949-42-9
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Beta-Lipotropin (1-10), porcine
Catalog No.:BCC1009
CAS No.:77875-68-4
- Isopulegol
Catalog No.:BCN4974
CAS No.:7786-67-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- (1R)-(+)-Alpha-Pinene
Catalog No.:BCC8275
CAS No.:7785-70-8
- Nelumol A
Catalog No.:BCN4749
CAS No.:77836-86-3
- GNF 5
Catalog No.:BCC3892
CAS No.:778277-15-9
- Linalool
Catalog No.:BCN6339
CAS No.:78-70-6
- Zeylasterone
Catalog No.:BCN8057
CAS No.:78012-25-6
- 1,3-Diacetylvilasinin
Catalog No.:BCN4580
CAS No.:78012-28-9
- MMPX
Catalog No.:BCC6692
CAS No.:78033-08-6
- Fmoc-Lys(Fmoc)-OH
Catalog No.:BCC3521
CAS No.:78081-87-5
- (R)-(+)-8-Hydroxy-DPAT hydrobromide
Catalog No.:BCC6929
CAS No.:78095-19-9
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
- Aztreonam
Catalog No.:BCC2557
CAS No.:78110-38-0
- Okadaic acid
Catalog No.:BCC2464
CAS No.:78111-17-8
- DAMGO
Catalog No.:BCC6958
CAS No.:78123-71-4
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- CDPPB
Catalog No.:BCC7610
CAS No.:781652-57-1
t-Butyl-Oxycarbonylated Diamines as Building Blocks for Isocyanate-Free Polyurethane/Urea Dispersions and Coatings.[Pubmed:29516566]
Macromol Rapid Commun. 2018 May;39(9):e1800004.
t-Butyl-oxycarbonylated diamines ("di-Boc-carbamates") are investigated as dicarbamate monomers for diamine/dicarbamate polymerizations. Polyureas (PUs) and polyurethanes (PURs) with high molecular weights are prepared from stoichiometric polymerizations of diamines or diols with N-N'-di-t-butyl-oxycarbonyl Isophorone diamine (DiBoc-IPDC) using KOt-Bu as a catalyst, while gelation is observed when an excess of DiBoc-IPDC is used with respect to the diamines or diols. Stable dispersions are obtained from PUs and PURs with 3,3'-diamino-N-methyldipropylamine (DMDPA) as internal dispersing agent. The corresponding PU-based coatings exhibit superior mechanical properties and solvent resistances compared to the polyurethane urea coatings synthesized from diols, DiBoc-IPDC, and DMDPA.
Evaluation of storage time effect on saffron chemical profile using gas chromatography and spectrophotometry techniques coupled with chemometrics.[Pubmed:29606749]
J Food Sci Technol. 2018 Apr;55(4):1350-1359.
Saffron quality is commonly determined by three parameters: color, aroma, and taste. Several factors including harvesting and post-harvesting conditions, affect these parameters. In this study, the effect of storage time on saffron quality was evaluated. At first, the relative concentration of the saffron secondary metabolites in freshly dried and 2 years stored saffron samples prepared with ISO 3632 and UA-DLLME methods and then measured using UV-Vis and GC-FID techniques. In order to find saffron storage time biomarkers, the obtained data were subjected to several data analysis steps including data preprocessing, principal component analysis (PCA), partial least square discriminant analysis (PLS-DA) and variable selection methods. Based on the obtained main biomarkers and proposed molecule mechanism, it can be concluded that during the storage periods, the intensity of saffron color reduces, while its aroma increases, reflecting a negative correlation between them. Freshly dried samples have a higher level of the crocins as coloring agents, beta-Isophorone, 4-hydroxy-3,5,5-trimethylcyclohex-2-enone and picrocrocin, while the stored samples were more abundant by safranal as the main saffron aroma agent.
Morphology and surface properties of high strength siloxane poly(urethane-urea)s developed for heart valve application.[Pubmed:29504237]
J Biomed Mater Res B Appl Biomater. 2019 Jan;107(1):112-121.
A series of siloxane poly(urethane-urea) (SiPUU) were developed by incorporating a macrodiol linked with a diisocyanate to enhance mixing of hard and soft segments (SS). The effect of this modification on morphology, surface properties, surface elemental composition, and creep resistance was investigated. The linked macrodiol was prepared by reacting alpha,omega-bis(6-hydroxyethoxypropyl) poly(dimethylsiloxane)(PDMS) or poly(hexamethylene oxide) (PHMO) with either 4,4'-methylenediphenyl diisocyanate (MDI), hexamethylene diisocyanate (HDI), or Isophorone diisocyanate (IPDI). SiPUU with PHMO-MDI-PHMO and PHMO-IPDI-PHMO linked macrodiols showed enhanced creep resistance and recovery when compared with a commercial biostable polyurethane, Elast-Eon 2A. Small and wide-angle X-ray scattering data were consistent with significant increase of hydrogen bonding between hard and SS with linked-macrodiols, which improved SiPUU's tensile stress and tear strengths. These SiPUU were hydrophobic with contact angle higher than 101 degrees and they had low water uptake (0.7%.w/w of dry mass). They also had much higher siloxane concentration on the surface compared to that in the bulk. (c) 2018 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 107B: 112-121, 2019.
Investigation on the mechanical properties of polyurea (PU)/melamine formaldehyde (MF) microcapsules prepared with different chain extenders.[Pubmed:29630422]
J Microencapsul. 2018 May;35(3):219-228.
There is lack of understanding on controlling of mechanical properties of moisture-curing PU/MF microcapsules which limited its further application. PU/MF microcapsules containing a core of Isophorone diisocyanate (IPDI) were prepared with different chain extenders, polyetheramine D400, H2O, triethylenetetramine and polyetheramine (PEA) D230 by following a two-step synthesis method in this study. Fourier transform infra-red (FTIR) spectroscopy, Malvern particle sizing, scanning electron microscopy (SEM), and transmission electron microscopy (TEM). And micromanipulation technique was used to identify chemical bonds in the shell, size distributions, structure, thickness, and mechanical properties of microcapsules. The results show that PU/MF microcapsules were successfully prepared. Tr increased from 46.4 +/- 13.9 N/m to 75.8 +/- 23.3 N/m when extender changed from D400 to D230. And the Tr increased from 51.3 +/- 14.1 to 94.8 +/- 17.5 N/m when the swelling time increased from 1 to 3h. Morphologies of the shell were utilised to understand the mechanism of reactions in forming the shell materials.
An integrated approach combining HPLC, GC/MS, NIRS, and chemometrics for the geographical discrimination and commercial categorization of saffron.[Pubmed:29502833]
Food Chem. 2018 Jul 1;253:284-292.
In the present study, an integrated approach combining HPLC/DAD, GC/MS, near infrared (NIR) spectroscopy, and chemometrics was used to geographically discriminate saffron samples from Iran and China. Chinese and Iranian samples can be well-separated based on HPLC data analysed by a principal component analysis and an orthogonal partial least squares discriminant analysis. Picrocrocin and two types of crocins were found to be the discriminating variables, and the Chinese samples had higher contents of safranal and picrocrocin but lower cis-crocin 3Gg, kaempferol-3-O-sophoroside and Isophorone. Furthermore, an NIR method was successfully established to rapidly distinguish the Chinese and Iranian samples. The relationship between an ISO standard and the contents of the chemical indices was also studied. The results indicated that the ISO standard should be revised, especially for analysing safranal.
Quantification of organic solvents in aquatic toys and swimming learning devices and evaluation of their influence on the smell properties of the corresponding products.[Pubmed:29464272]
Anal Bioanal Chem. 2018 Apr;410(10):2585-2595.
Based on the observation that the characteristic odour of inflatable aquatic toys for children is predominantly caused by residues of hazardous organic solvents, the concentrations of cyclohexanone, Isophorone and phenol were determined in a selection of 20 products obtained from online suppliers located in Germany. Analytes were extracted with dichloromethane after the addition of non-labelled internal standards, and the volatile fraction was isolated using solvent-assisted flavour evaporation (SAFE). Extracts were then concentrated by Vigreux distillation and analysed by means of gas chromatography with mass spectrometric detection (GC-MS). Furthermore, each sample was evaluated regarding its specific olfactory properties by an expert sensory panel. While some samples did not contain significant amounts of solvents, cyclohexanone concentrations above the lower limit of quantification (LLOQ) were determined in nine samples with six samples containing high concentrations ranging from about 1 to 7 g/kg cyclohexanone. Isophorone concentrations above the LLOQ were observed in eight samples. Thereby, six products contained between 0.3 and 1.6 g/kg Isophorone and the remaining two samples contained even about 5 g/kg Isophorone, each. Likewise, phenol concentrations exceeded the LLOQ in 14 cases, with four samples containing elevated amounts ranging from about 140 to 280 mg/kg phenol.
The Production of Methane, Acetone, "Cold" CO and Oxygenated Species from IsoPropyl Alcohol in a Non-Thermal Plasma: An In-Situ FTIR Study.[Pubmed:29664640]
J Phys Chem A. 2018 May 3;122(17):4273-4284.
This paper reports in situ Fourier transform infrared (FTIR) spectroscopic studies on the nonthermal plasma reaction of isopropyl alcohol in dinitrogen at Macor (a ceramic containing oxides of Al, Mg, and Si) and the analogous thermally driven process. While isopropyl alcohol did not react at the Macor at temperatures up to 600 degrees C, the study of the nonthermal plasma-driven process at the ceramic led to unexpected chemistry hitherto not observed, primarily the reaction of IPA in dinitrogen at short time scales to produce methane, HCN, acetone and "cold" CO at ca. 115 K. The CO, methane, and HCN rapidly established steady state concentrations, pointing to the need for faster FTIR studies: at longer times, Isophorone and a "polymethylacetylene-like" polymer were formed as a brown oil. The observation of the steady-state gases and brown oil suggested parallel pathways in the plasma, the latter taking place at the plasma/catalyst interface, and the former in the plasma remote from the catalyst. Replacing dinitrogen with argon completely inhibited or negated the production of the oil, had no effect upon the processes taking place in the plasma remote from the Macor, and instead resulted in the production of acetylene.
Isophorone Diisocyanate: An Effective Additive to Form Cathode-Protective-Interlayer and Its Influence on LiNi0.5Co0.2Mn0.3O2 at High Potential.[Pubmed:29547257]
ACS Appl Mater Interfaces. 2018 Apr 4;10(13):11305-11310.
In this work, we propose a novel electrolyte additive, Isophorone diisocyanate (IPDI), to construct the surface protective interlayer. This membrane is produced via nucleophilic addition between the IPDI's diisocyanate groups and the free-radical-onium ion oxidative intermediate of propylene carbonate (PC). In the electrolyte with IPDI added between 10-20 mM, LiNi0.5Co0.2Mn0.3O2 presents the excellent performance, demonstrating the relative wide operational window to form the optimal protective membrane. This protective membrane ameliorates the cyclic stability. Although all systems deliver approximately 185 mAh g(-1) under 1 C between 2.5-4.6 V (vs Li(+)/Li), the cells in the suitable electrolyte maintain 90.4% in the 50 cycles and 83.2% in the 200 cycles, whereas the control cells are seriously dropped to 73.4% and 69.8%. The cells in the electrolyte with the appropriate IPDI also present the good rate capability, attaining approximately 143 mAh g(-1) under 5 C, much higher than the cells in the control electrolyte(92.4 mAh g(-1)). The additive proposed in this work is helpful to augment the energy density of lithium ion battery and prolong the one-drive distance of electric vehicles.


