glucagon receptor antagonists 1Glucagon receptor antagonist,highly potent CAS# 503559-84-0 |
- Exendin-4
Catalog No.:BCC1568
CAS No.:141758-74-9
- glucagon receptor antagonists 3
Catalog No.:BCC1595
CAS No.:202917-17-7
- glucagon receptor antagonists 2
Catalog No.:BCC1594
CAS No.:202917-18-8
- MK 0893
Catalog No.:BCC1752
CAS No.:870823-12-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
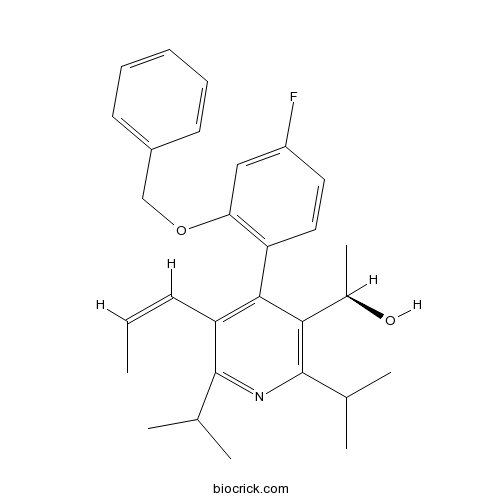
| Cas No. | 503559-84-0 | SDF | Download SDF |
| PubChem ID | 97041483 | Appearance | Powder |
| Formula | C29H34FNO2 | M.Wt | 447.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (111.71 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (1R)-1-[4-(4-fluoro-2-phenylmethoxyphenyl)-2,6-di(propan-2-yl)-5-[(Z)-prop-1-enyl]pyridin-3-yl]ethanol | ||
| SMILES | CC=CC1=C(C(=C(N=C1C(C)C)C(C)C)C(C)O)C2=C(C=C(C=C2)F)OCC3=CC=CC=C3 | ||
| Standard InChIKey | HERVQUFQZXZOBU-RVPHRTOXSA-N | ||
| Standard InChI | InChI=1S/C29H34FNO2/c1-7-11-24-27(26(20(6)32)29(19(4)5)31-28(24)18(2)3)23-15-14-22(30)16-25(23)33-17-21-12-9-8-10-13-21/h7-16,18-20,32H,17H2,1-6H3/b11-7-/t20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glucagon receptor antagonists-1 is a highly potent glucagon receptor antagonist. References: | |||||

glucagon receptor antagonists 1 Dilution Calculator

glucagon receptor antagonists 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2341 mL | 11.1707 mL | 22.3414 mL | 44.6828 mL | 55.8534 mL |
| 5 mM | 0.4468 mL | 2.2341 mL | 4.4683 mL | 8.9366 mL | 11.1707 mL |
| 10 mM | 0.2234 mL | 1.1171 mL | 2.2341 mL | 4.4683 mL | 5.5853 mL |
| 50 mM | 0.0447 mL | 0.2234 mL | 0.4468 mL | 0.8937 mL | 1.1171 mL |
| 100 mM | 0.0223 mL | 0.1117 mL | 0.2234 mL | 0.4468 mL | 0.5585 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
highly potent glucagon receptor antagonist
- NU7441 (KU-57788)
Catalog No.:BCC3679
CAS No.:503468-95-9
- 3'-Galloylquercitrin
Catalog No.:BCN8254
CAS No.:503446-90-0
- N-Methylflindersine
Catalog No.:BCN3641
CAS No.:50333-13-6
- Vilanterol trifenatate
Catalog No.:BCC4031
CAS No.:503070-58-4
- Vilanterol
Catalog No.:BCC4030
CAS No.:503068-34-6
- Boc-His(Z)-OH
Catalog No.:BCC3404
CAS No.:50305-43-6
- Cucurbitacin IIb
Catalog No.:BCN2519
CAS No.:50298-90-3
- Erythrocentaurin
Catalog No.:BCN7684
CAS No.:50276-98-7
- Oleuropeic acid
Catalog No.:BCN5611
CAS No.:5027-76-9
- Phloracetophenone 4'-O-glucoside
Catalog No.:BCN4052
CAS No.:5027-30-5
- Lonidamine
Catalog No.:BCC9012
CAS No.:50264-69-2
- SQ109
Catalog No.:BCC1962
CAS No.:502487-67-4
- Pentamidine dihydrochloride
Catalog No.:BCC5194
CAS No.:50357-45-4
- Apixaban
Catalog No.:BCC2295
CAS No.:503612-47-3
- Cyclo(Phe-Gly)
Catalog No.:BCN2431
CAS No.:5037-75-2
- (-)-di-de-Omethylgrandisin
Catalog No.:BCN7872
CAS No.:50393-98-1
- Orcinol
Catalog No.:BCN5612
CAS No.:504-15-4
- 4-Aminopyridine
Catalog No.:BCC5267
CAS No.:504-24-5
- DL-Homocysteic acid
Catalog No.:BCN2233
CAS No.:504-33-6
- 3-Nitropropionic acid
Catalog No.:BCC6303
CAS No.:504-88-1
- Juglanin
Catalog No.:BCN6505
CAS No.:5041-67-8
- Isoliquiritin
Catalog No.:BCN5945
CAS No.:5041-81-6
- Isorhamnetin-3-O-beta-D-Glucoside
Catalog No.:BCN1247
CAS No.:5041-82-7
- 1,5,6-Trihydroxyxanthone
Catalog No.:BCN7642
CAS No.:5042-03-5
Discovery of a Novel Series of Orally Bioavailable and CNS Penetrant Glucagon-like Peptide-1 Receptor (GLP-1R) Noncompetitive Antagonists Based on a 1,3-Disubstituted-7-aryl-5,5-bis(trifluoromethyl)-5,8-dihydropyrimido[4,5-d]pyrim idine-2,4(1H,3H)-dione Core.[Pubmed:28103022]
J Med Chem. 2017 Feb 23;60(4):1611-1616.
A duplexed, functional multiaddition high throughput screen and subsequent optimization effort identified the first orally bioavailable and CNS penetrant glucagon-like peptide-1 receptor (GLP-1R) noncompetitive antagonist. Antagonist 5d not only blocked exendin-4-stimulated insulin release in islets but also lowered insulin levels while increasing blood glucose in vivo.
desHis(1)Glu(9)-glucagon-[mPEG] and desHis(1)Glu(9)(Lys(3)(0)PAL)-glucagon: long-acting peptide-based PEGylated and acylated glucagon receptor antagonists with potential antidiabetic activity.[Pubmed:23562625]
Eur J Pharmacol. 2013 Jun 5;709(1-3):43-51.
Glucagon is hormone secreted from the pancreatic alpha-cells that is involved in blood glucose regulation. As such, antagonism of glucagon receptor signalling represents an exciting approach for treating diabetes. To harness these beneficial metabolic effects, two novel glucagon analogues, desHis(1)Glu(9)-glucagon-[mPEG] and desHis(1)Glu(9)(Lys(3)(0)PAL)-glucagon, has been evaluated for potential glucagon receptor antagonistic properties. Both novel peptides were completely resistant to enzymatic breakdown and significantly (P<0.05 to P<0.001) inhibited glucagon-mediated elevations of cAMP production in glucagon receptor transfected cells. Similarly, desHis(1)Glu(9)-glucagon-[mPEG] and desHis(1)Glu(9)(Lys(3)(0)PAL)-glucagon effectively antagonised glucagon-induced increases of insulin secretion from BRIN BD11 cells. When administered acutely to normal, high fat fed or ob/ob mice, both analogues had no significant effects on overall blood glucose or plasma insulin levels when compared to saline treated controls. However, desHis(1)Glu(9)-glucagon-[mPEG] significantly (P<0.05) annulled glucagon-induced increases in blood glucose and plasma insulin levels in normal mice and had similar non-significant tendencies in high fat and ob/ob mice. In addition, desHis(1)Glu(9)(Lys(3)(0)PAL)-glucagon effectively (P<0.05 to P<0.001) antagonised glucagon-mediated elevations of blood glucose levels in high fat fed and ob/ob mice, but was less efficacious in normal mice. Further studies confirmed the significant persistent glucagon receptor antagonistic properties of both novel enzyme-resistant analogues 4h post administration in normal mice. These studies emphasise the potential of longer-acting peptide-based glucagon receptor antagonists, and particularly acylated versions, for the treatment of diabetes.
Optimization of alkylidene hydrazide based human glucagon receptor antagonists. Discovery of the highly potent and orally available 3-cyano-4-hydroxybenzoic acid [1-(2,3,5,6-tetramethylbenzyl)-1H-indol-4-ylmethylene]hydrazide.[Pubmed:12477359]
J Med Chem. 2002 Dec 19;45(26):5755-75.
Highly potent human glucagon receptor (hGluR) antagonists have been prepared employing both medicinal chemistry and targeted libraries based on modification of the core (proximal) dimethoxyphenyl group, the benzyl ether linkage, as well as the (distal) benzylic aryl group of the lead 2, 3-cyano-4-hydroxybenzoic acid (3,5-dimethoxy-4-isopropylbenzyloxybenzylidene)hydrazide. Electron-rich proximal aryl moieties such as mono- and dimethoxy benzenes, naphthalenes, and indoles were found to be active. The SAR was found to be quite insensitive regarding the linkage to the distal aryl group, since long and short as well as polar and apolar linkers gave highly potent compounds. The presence of a distal aryl group was not crucial for obtaining high binding affinity to the hGluR. In many cases, however, the affinity could be further optimized with substituted distal aryl groups. Representative compounds have been tested for in vitro metabolism, and structure-metabolism relationships are described. These efforts lead to the discovery of 74, NNC 25-2504, 3-cyano-4-hydroxybenzoic acid [1-(2,3,5,6-tetramethylbenzyl)-1H-indol-4-ylmethylene]hydrazide, with low in vitro metabolic turnover. 74 was a highly potent noncompetitive antagonist of the human glucagon receptor (IC(50) = 2.3 nM, K(B) = 760 pM) and of the isolated rat receptor (IC(50) = 430 pM, K(B) = 380 pM). Glucagon-stimulated glucose production from isolated primary rat hepatocytes was inhibited competitively by 74 (K(i) = 14 nM). This compound was orally available in dogs (F(po) = 15%) and was active in a glucagon-challenged rat model of hyperglucagonemia and hyperglycemia.
High potency antagonists of the pancreatic glucagon-like peptide-1 receptor.[Pubmed:9261127]
J Biol Chem. 1997 Aug 22;272(34):21201-6.
GLP-1-(7-36)-amide and exendin-4-(1-39) are glucagon-like peptide-1 (GLP-1) receptor agonists, whereas exendin-(9-39) is the only known antagonist. To analyze the transition from agonist to antagonist and to identify the amino acid residues involved in ligand activation of the GLP-1 receptor, we used exendin analogs with successive N-terminal truncations. Chinese hamster ovary cells stably transfected with the rat GLP-1 receptor were assayed for changes in intracellular cAMP caused by the test peptides in the absence or presence of half-maximal stimulatory doses of GLP-1. N-terminal truncation of a single amino acid reduced the agonist activity of the exendin peptide, whereas N-terminal truncation of 3-7 amino acids produced antagonists that were 4-10-fold more potent than exendin-(9-39). N-terminal truncation of GLP-1 by 2 amino acids resulted in weak agonist activity, but an 8-amino acid N-terminal truncation inactivated the peptide. Binding studies performed using 125I-labeled GLP-1 confirmed that all bioactive peptides specifically displaced tracer with high potency. In a set of exendin/GLP-1 chimeric peptides, substitution of GLP-1 sequences into exendin-(3-39) produced loss of antagonist activity with conversion to a weak agonist. The results show that receptor binding and activation occur in separate domains of exendin, but they are more closely coupled in GLP-1.


