Tyrosine kinase inhibitorTyrosine-kinase inhibitor CAS# 1021950-26-4 |
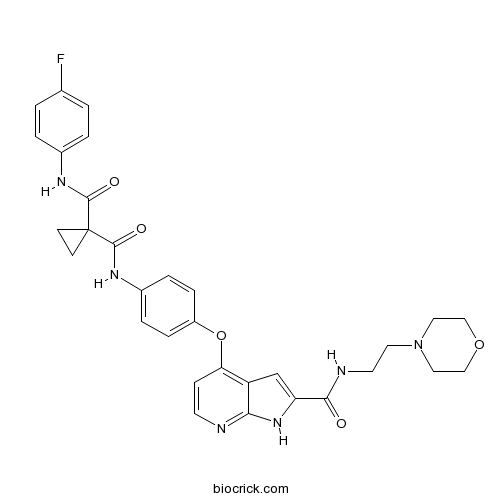
- AMG-208
Catalog No.:BCC1054
CAS No.:1002304-34-8
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- SU11274
Catalog No.:BCC1243
CAS No.:658084-23-2
- Cabozantinib (XL184, BMS-907351)
Catalog No.:BCC1264
CAS No.:849217-68-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1021950-26-4 | SDF | Download SDF |
| PubChem ID | 24956525 | Appearance | Powder |
| Formula | C31H31FN6O5 | M.Wt | 586.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 46 mg/mL (78.42 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 1-N'-(4-fluorophenyl)-1-N-[4-[[2-(2-morpholin-4-ylethylcarbamoyl)-1H-pyrrolo[2,3-b]pyridin-4-yl]oxy]phenyl]cyclopropane-1,1-dicarboxamide | ||
| SMILES | C1CC1(C(=O)NC2=CC=C(C=C2)OC3=C4C=C(NC4=NC=C3)C(=O)NCCN5CCOCC5)C(=O)NC6=CC=C(C=C6)F | ||
| Standard InChIKey | PKOVTRMHYNEBDU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H31FN6O5/c32-20-1-3-21(4-2-20)35-29(40)31(10-11-31)30(41)36-22-5-7-23(8-6-22)43-26-9-12-33-27-24(26)19-25(37-27)28(39)34-13-14-38-15-17-42-18-16-38/h1-9,12,19H,10-11,13-18H2,(H,33,37)(H,34,39)(H,35,40)(H,36,41) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A Tyrosine kinase inhibitor. References: | |||||

Tyrosine kinase inhibitor Dilution Calculator

Tyrosine kinase inhibitor Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7047 mL | 8.5236 mL | 17.0471 mL | 34.0942 mL | 42.6178 mL |
| 5 mM | 0.3409 mL | 1.7047 mL | 3.4094 mL | 6.8188 mL | 8.5236 mL |
| 10 mM | 0.1705 mL | 0.8524 mL | 1.7047 mL | 3.4094 mL | 4.2618 mL |
| 50 mM | 0.0341 mL | 0.1705 mL | 0.3409 mL | 0.6819 mL | 0.8524 mL |
| 100 mM | 0.017 mL | 0.0852 mL | 0.1705 mL | 0.3409 mL | 0.4262 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A tyrosine-kinase inhibitor (TKI) is a pharmaceutical drug that inhibits tyrosine kinases. Tyrosine kinases are enzymes responsible for the activation of many proteins by signal transduction cascades. The proteins are activated by adding a phosphate group
- Boc-4-oxo-Pro-OMe
Catalog No.:BCC3436
CAS No.:102195-80-2
- 3-O-Methyltirotundin
Catalog No.:BCN5837
CAS No.:1021945-29-8
- ZM 39923 HCl
Catalog No.:BCC2203
CAS No.:1021868-92-7
- AGN 192403 hydrochloride
Catalog No.:BCC6924
CAS No.:1021868-90-5
- ARL 67156 trisodium salt
Catalog No.:BCC7004
CAS No.:1021868-83-6
- PPNDS
Catalog No.:BCC7015
CAS No.:1021868-77-8
- Boc-D-N-Me-Phe.DCHA
Catalog No.:BCC3347
CAS No.:102185-45-5
- Boc-Arg(Mts)-OH
Catalog No.:BCC3054
CAS No.:102185-38-6
- Boc-D-Pro-OSu
Catalog No.:BCC3438
CAS No.:102185-34-2
- DPCPX
Catalog No.:BCC6649
CAS No.:102146-07-6
- RJR 2429 dihydrochloride
Catalog No.:BCC7000
CAS No.:1021418-53-0
- Neoprocurcumenol
Catalog No.:BCN3694
CAS No.:102130-91-6
- ARN2966
Catalog No.:BCC8074
CAS No.:102212-26-0
- Btk inhibitor 1 R enantiomer
Catalog No.:BCC5125
CAS No.:1022150-12-4
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- Epoxyparvinolide
Catalog No.:BCN5838
CAS No.:102227-61-2
- (R)-Eriodictyol-8-C-beta-D-glucopyranoside
Catalog No.:BCN8028
CAS No.:1023271-51-3
- Cadensin D
Catalog No.:BCN7260
CAS No.:102349-35-9
- Naringin
Catalog No.:BCN6312
CAS No.:10236-47-2
- Negsehisandrin G
Catalog No.:BCN2674
CAS No.:1023744-69-5
- Glyburide
Catalog No.:BCC4784
CAS No.:10238-21-8
- AF-DX 116
Catalog No.:BCC6939
CAS No.:102394-31-0
- Jasplakinolide
Catalog No.:BCC7485
CAS No.:102396-24-7
- Fmoc-Phg-OH
Catalog No.:BCC3312
CAS No.:102410-65-1
Taiwanese Dermatological Association consensus for the prevention and management of epidermal growth factor receptor tyrosine kinase inhibitor-related skin toxicities.[Pubmed:28351555]
J Formos Med Assoc. 2017 Jun;116(6):413-423.
BACKGROUND/PURPOSE: This report describes the 2016 consensus of the Taiwanese Dermatological Association (TDA) regarding the definition, classification, diagnosis, prevention, and management of skin toxicities resulting from treatment with epidermal growth factor receptor (EGFR) Tyrosine kinase inhibitors (TKIs). This consensus is distributed to practices throughout Taiwan to provide recommendations for the diagnosis and treatment of such skin toxicities in order to improve the quality of life of patients undergoing EGFR-TKI treatment. The consensus thus serves as an important reference for dermatologists and other interested clinicians, such as oncologists, throughout Taiwan. METHODS: All the consensus contents were voted on by the participating experts, with approval by no less than 75% required for inclusion. RESULTS: The consensus provides a comprehensive overview of EGFR-TKI skin toxicities, including recent advances in identifying their causes and the processes by which they develop. CONCLUSION: All the consensus meeting attendees agreed that there are several major EGFR-TKI-related skin toxicities, including acneiform rash (i.e., papulopustular rash), xeroderma, pruritus, paronychia, stomatitis, mucositis, and hair changes (such as hair loss, slowed hair growth, and trichomegaly). The experts were also generally unanimous in their voting on the specific definitions, onset times, and care suggestions for each of those skin toxicities. Furthermore, the recommended treatment algorithms for the various skin toxicities were ultimately approved by 100% (15/15) of the consensus attendees.
JAK2 tyrosine kinase inhibitor AG490 suppresses cell growth and invasion of gallbladder cancer cells via inhibition of JAK2/STAT3 signaling.[Pubmed:28337870]
J Biol Regul Homeost Agents. 2017 Jan-Mar;31(1):51-58.
The Janus kinase-signal transducers and activators of transcription signaling pathway (JAK/STAT pathway) have displayed a critical role in tumor development and progression in multiple malignancies. Previous studies showed that inhibition of JAK/STAT signaling blocked cell growth and metastasis in cancer cells, however, the antitumor effects of JAK inhibitor AG490 on gallbladder cancer (GBC) have not been reported. Our present study aimed to investigate the effects and associated mechanisms of JAK inhibitor AG490 on cell growth, invasive potential and apoptosis in GBC cells (GBC-SD and SGC-996) indicated by MTT, cell colony formation, Transwell and flow cytometry. As a consequence, we found that JAK2 inhibitor AG490 inhibited cell growth and invasion, and induced cell apoptosis and cycle arrest in GBC-SD and SGC-996 cells. Furthermore, the expression levels of p-JAK2, p-STAT3, VEGFC-/-D and cyclinD1 were downregulated, while p53 expression was upregulated in AG490-treated GBC cells indicated by Western blot assay. Therefore, our findings demonstrate that JAK inhibitor AG490 inhibits growth and invasion of GBC cells via blockade of JAK2/STAT3 signaling and provides the potential therapeutic strategy for the treatment of GBC patients.
Tyrosine kinase inhibitor therapy-induced changes in humoral immunity in patients with chronic myeloid leukemia.[Pubmed:28337541]
J Cancer Res Clin Oncol. 2017 Aug;143(8):1543-1554.
PURPOSE: Tyrosine kinase inhibitors (TKIs) have well-characterized immunomodulatory effects on T and NK cells, but the effects on the humoral immunity are less well known. In this project, we studied TKI-induced changes in B cell-mediated immunity. METHODS: We collected peripheral blood (PB) and bone marrow (BM) samples from chronic myeloid leukemia (CML) patients before and during first-line imatinib (n = 20), dasatinib (n = 16), nilotinib (n = 8), and bosutinib (n = 12) treatment. Plasma immunoglobulin levels were measured, and different B cell populations in PB and BM were analyzed with flow cytometry. RESULTS: Imatinib treatment decreased plasma IgA and IgG levels, while dasatinib reduced IgM levels. At diagnosis, the proportion of patients with IgA, IgG, and IgM levels below the lower limit of normal (LLN) was 0, 11, and 6% of all CML patients, respectively, whereas at 12 months timepoint the proportions were 6% (p = 0.13), 31% (p = 0.042) and 28% (p = 0.0078). Lower initial Ig levels predisposed to the development of hypogammaglobulinemia during TKI therapy. Decreased Ig levels in imatinib-treated patients were associated with higher percentages of immature BM B cells. The patients, who had low Ig levels during the TKI therapy, had significantly more frequent minor infections during the follow-up compared with the patients with normal Ig values (33% vs. 3%, p = 0.0016). No severe infections were reported, except recurrent upper respiratory tract infections in one imatinib-treated patient, who developed severe hypogammaglobulinemia. CONCLUSIONS: TKI treatment decreases plasma Ig levels, which should be measured in patients with recurrent infections.
Does the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor differ according to the type of EGFR mutation in non-small cell lung cancer?[Pubmed:28352061]
Korean J Intern Med. 2017 May;32(3):422-428.
An exon 19 deletion and a L858R mutation in exon 21 of the epidermal growth factor receptor (EGFR) are the two most common mutations that predict favorable efficacy of EGFR Tyrosine kinase inhibitors (TKIs) in patients with non-small cell lung cancer (NSCLC). Many retrospective and prospective studies, as well as meta-analyses including patients with NSCLC with various lines of EGFR TKI treatment, have demonstrated longer progression-free survival and sometimes more favorable overall survival in patients with an exon 19 deletion than those with the L858R or other mutations. In contrast, some clinical studies, including phase III trials, have demonstrated no difference in the efficacy of EGFR TKIs according to the EGFR mutation type. Therefore, the existence of clinically significant differences in sensitivity to EGFR-TKIs among different EGFR mutation subtypes remains controversial. In this review, we summarize the evidence suggesting different outcomes according to the type of EGFR mutation in patients with advanced NSCLC who were treated with EGFR-TKIs, along with their clinical significance. We also discuss possible mechanisms that can explain the different sensitivities to EGFR TKIs between cases with an exon 19 deletion and those with the L858R mutation.


