GlyburideKir6 (KATP) channel blocker CAS# 10238-21-8 |
- Betamethasone
Catalog No.:BCC4765
CAS No.:378-44-9
- Beclomethasone dipropionate
Catalog No.:BCC4257
CAS No.:5534-09-8
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
- Mifepristone
Catalog No.:BCC4486
CAS No.:84371-65-3
- Betamethasone hydrochloride
Catalog No.:BCC4256
CAS No.:956901-32-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 10238-21-8 | SDF | Download SDF |
| PubChem ID | 3488 | Appearance | Powder |
| Formula | C23H28ClN3O5S | M.Wt | 494 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Glyburide | ||
| Solubility | DMSO : ≥ 60 mg/mL (121.46 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
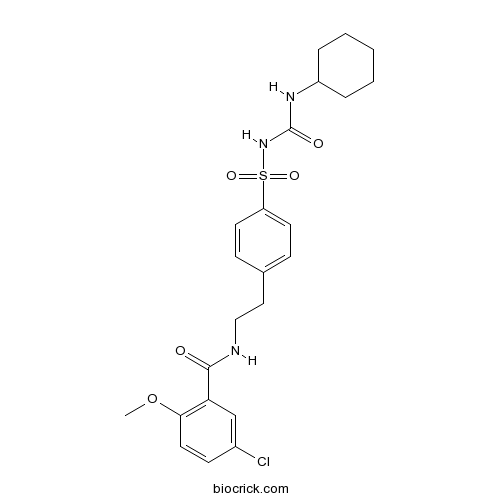
| Chemical Name | 5-chloro-N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-2-methoxybenzamide | ||
| SMILES | COC1=C(C=C(C=C1)Cl)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCCCC3 | ||
| Standard InChIKey | ZNNLBTZKUZBEKO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glibenclamide is a known blocker of vascular ATP-sensitive K+ channels (KATP), used in the treatment of type 2 diabetes.Glibenclamide increases the risk for hypoglycemia by increasing insulin secretion, it plays a paradoxical protective role to protect against severe hypoglycemia-induced fatal cardiac arrhythmias; it also reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. |
| Targets | TNF-α | NF-kB | Caspase | ATPase | Potassium Channel |
| In vivo | Glibenclamide, but not acarbose, increases leptin concentrations parallel to changes in insulin in subjects with NIDDM.[Pubmed: 9283792]Diabetes Care. 1997 Sep;20(9):1430-4.To hypothesize if Glibenclamide, which increases insulin levels, also increases leptin concentrations.
Inhibition by glibenclamide of the vasorelaxant action of cromakalim in the rat.[Pubmed: 2497925]Br J Pharmacol. 1989 May;97(1):57-64.
Glibenclamide Prevents Hypoglycemia-Induced Fatal Cardiac Arrhythmias in Rats.[Pubmed: 29800118 ]Endocrinology. 2018 Jul 1;159(7):2614-2620.Sulfonylureas increase the incidence of severe hypoglycemia in people with type 2 diabetes and might increase the risk of sudden cardiac death. Sulfonylureas stimulate insulin secretion by closing pancreatic ATP-sensitive potassium ion (KATP) channels. |
| Kinase Assay | Glibenclamide is a competitive antagonist of cromakalim, pinacidil and RP 49356 in guinea-pig pulmonary artery.[Pubmed: 2528466]Eur J Pharmacol. 1989 Jun 20;165(2-3):231-9.
|
| Animal Research | Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage[Pubmed: 18854840 ]J Cereb Blood Flow Metab. 2009 Feb; 29(2): 317–330.Subarachnoid hemorrhage (SAH) causes secondary brain injury due to vasospasm and inflammation. |

Glyburide Dilution Calculator

Glyburide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0243 mL | 10.1215 mL | 20.2429 mL | 40.4858 mL | 50.6073 mL |
| 5 mM | 0.4049 mL | 2.0243 mL | 4.0486 mL | 8.0972 mL | 10.1215 mL |
| 10 mM | 0.2024 mL | 1.0121 mL | 2.0243 mL | 4.0486 mL | 5.0607 mL |
| 50 mM | 0.0405 mL | 0.2024 mL | 0.4049 mL | 0.8097 mL | 1.0121 mL |
| 100 mM | 0.0202 mL | 0.1012 mL | 0.2024 mL | 0.4049 mL | 0.5061 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Glyburide
- Negsehisandrin G
Catalog No.:BCN2674
CAS No.:1023744-69-5
- Naringin
Catalog No.:BCN6312
CAS No.:10236-47-2
- Cadensin D
Catalog No.:BCN7260
CAS No.:102349-35-9
- (R)-Eriodictyol-8-C-beta-D-glucopyranoside
Catalog No.:BCN8028
CAS No.:1023271-51-3
- Epoxyparvinolide
Catalog No.:BCN5838
CAS No.:102227-61-2
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- Btk inhibitor 1 R enantiomer
Catalog No.:BCC5125
CAS No.:1022150-12-4
- ARN2966
Catalog No.:BCC8074
CAS No.:102212-26-0
- Tyrosine kinase inhibitor
Catalog No.:BCC2020
CAS No.:1021950-26-4
- Boc-4-oxo-Pro-OMe
Catalog No.:BCC3436
CAS No.:102195-80-2
- 3-O-Methyltirotundin
Catalog No.:BCN5837
CAS No.:1021945-29-8
- ZM 39923 HCl
Catalog No.:BCC2203
CAS No.:1021868-92-7
- AF-DX 116
Catalog No.:BCC6939
CAS No.:102394-31-0
- Jasplakinolide
Catalog No.:BCC7485
CAS No.:102396-24-7
- Fmoc-Phg-OH
Catalog No.:BCC3312
CAS No.:102410-65-1
- RN 1747
Catalog No.:BCC7769
CAS No.:1024448-59-6
- Kushenol N
Catalog No.:BCN2984
CAS No.:102490-65-3
- GK921
Catalog No.:BCC8057
CAS No.:1025015-40-0
- Periglaucine A
Catalog No.:BCN5839
CAS No.:1025023-04-4
- Periglaucine B
Catalog No.:BCN7053
CAS No.:1025023-05-5
- SGI-1776 free base
Catalog No.:BCC2232
CAS No.:1025065-69-3
- (-)-Huperzine A
Catalog No.:BCN1057
CAS No.:102518-79-6
- 2,3,23-Trihydroxy-12-oleanen-28-oic acid
Catalog No.:BCN1638
CAS No.:102519-34-6
- R788 disodium
Catalog No.:BCC3695
CAS No.:1025687-58-4
Inhibition by glibenclamide of the vasorelaxant action of cromakalim in the rat.[Pubmed:2497925]
Br J Pharmacol. 1989 May;97(1):57-64.
1. In rat isolated thoracic aortic rings pre-contracted with noradrenaline (10(-6) M), cromakalim (3 x 10(-7)-3 x 10(-5) M) produced concentration-related relaxation. This effect was progressively inhibited by increasing concentrations of the anti-diabetic sulphonylurea drug, glibenclamide (10(-6)-10(-5) M). 2. In rat isolated portal veins, cromakalim (3 x 10(-8)-10(-6) M) produced concentration-related inhibition of the spontaneous contractive activity and glibenclamide (3 x 10(-7)-3 x 10(-6) M) prevented this inhibitory action in a concentration-dependent manner. 3. In both rat aortic rings and portal veins, cromakalim (10(-5) M) stimulated 86Rb efflux. Prior exposure to glibenclamide (10(-7)-10(-6) M) produced a concentration-related inhibition of this response. 4. In conscious rats, cromakalim, 0.075 mg kg-1 i.v., produced a rapid and sustained fall in arterial blood pressure which was not influenced by pretreatment (2 h) with a large oral dose of glibenclamide (100 mg kg-1). 5. In conscious rats, the hypotensive action of cromakalim, 0.075 mg kg-1 i.v., was abolished by pretreatment (30 min) with glibenclamide, 20 mg kg-1, given by the intravenous route. 6. The results suggest that the vasorelaxant and hypotensive actions of cromakalim involve a K+ channel which can be inhibited by glibenclamide, but which may be distinct from the ATP-sensitive K+ channel of the pancreatic beta-cell.
Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage.[Pubmed:18854840]
J Cereb Blood Flow Metab. 2009 Feb;29(2):317-30.
Subarachnoid hemorrhage (SAH) causes secondary brain injury due to vasospasm and inflammation. Here, we studied a rat model of mild-to-moderate SAH intended to minimize ischemia/hypoxia to examine the role of sulfonylurea receptor 1 (SUR1) in the inflammatory response induced by SAH. mRNA for Abcc8, which encodes SUR1, and SUR1 protein were abundantly upregulated in cortex adjacent to SAH, where tumor-necrosis factor-alpha (TNFalpha) and nuclear factor (NF)kappaB signaling were prominent. In vitro experiments confirmed that Abcc8 transcription is stimulated by TNFalpha. To investigate the functional consequences of SUR1 expression after SAH, we studied the effect of the potent, selective SUR1 inhibitor, glibenclamide. We examined barrier permeability (immunoglobulin G, IgG extravasation), and its correlate, the localization of the tight junction protein, zona occludens 1 (ZO-1). SAH caused a large increase in barrier permeability and disrupted the normal junctional localization of ZO-1, with glibenclamide significantly reducing both effects. In addition, SAH caused large increases in markers of inflammation, including TNFalpha and NFkappaB, and markers of cell injury or cell death, including IgG endocytosis and caspase-3 activation, with glibenclamide significantly reducing these effects. We conclude that block of SUR1 by glibenclamide may ameliorate several pathologic effects associated with inflammation that lead to cortical dysfunction after SAH.
Glibenclamide is a competitive antagonist of cromakalim, pinacidil and RP 49356 in guinea-pig pulmonary artery.[Pubmed:2528466]
Eur J Pharmacol. 1989 Jun 20;165(2-3):231-9.
The relaxant effect of cromakalim (BRL 34915), pinacidil and RP 49356 (N-methyl-2-(3-pyridyl)-tetrahydro-thiopyran-2-carbothioamide-1-ox ide) on the sustained contractions induced by 20 mM KCl were compared with the effects of nicorandil. The preparation used was vascular smooth muscle of phenoxybenzamine-treated pulmonary artery rings from reserpinized guinea-pigs. Cromakalim, pinacidil, RP 49356 and nicorandil relaxed the tissues with -log EC50 values of 6.78, 6.12, 6.02 and 5.46, respectively. The inhibitory effect of cromakalim, pinacidil and RP 49356, but not of nicorandil, was competitively antagonized by glibenclamide (10(-7)-3 X 10(-6) M), yielding uniform pA2 values of 7.17-7.22 against all three relaxant drugs. The order of potency of other K+ channel blocking agents for the inhibition of vasorelaxation by cromakalim, pinacidil and RP 49356 was procaine greater than 4-aminopyridine greater than tetraethylammonium. The mainly competitive type of inhibition of the RP 49356-induced response was more comparable to that with pinacidil than with cromakalim. The relaxation caused by nicorandil was only attenuated by a high concentration of 4-aminopyridine or tetraethylammonium but was markedly antagonized by methylene blue (3 X 10(-6)-10(-5) M) and potentiated by M & B 22948 (3 X 10(-6)-10(-5) M). These results suggest that the vascular relaxation caused in guinea-pig pulmonary artery by cromakalim, pinacidil and RP 49356 is mediated through the same glibenclamide-sensitive K+ channel whereas the major mechanism for the effect of nicorandil seems to involve stimulation of guanylate cyclase.
Glibenclamide, but not acarbose, increases leptin concentrations parallel to changes in insulin in subjects with NIDDM.[Pubmed:9283792]
Diabetes Care. 1997 Sep;20(9):1430-4.
OBJECTIVE: To hypothesize if glibenclamide, which increases insulin levels, also increases leptin concentrations. RESEARCH DESIGN AND METHODS: Leptin is a hormone that regulates weight in mice. In obese humans, leptin concentrations are increased, suggesting resistance to the effects of this hormone. Although short-term infusion of insulin during the hyperinsulinemiceuglycemic clamp does not increase leptin concentration, the effect of oral antidiabetic agents on leptin concentration is unknown. Differing effects can be expected, since glibenclamide acts via stimulation of insulin secretion, whereas acarbose inhibits alpha-glucosidases of the small intestine and has no direct effect on insulin levels. We examined the effect of acarbose (n = 4), glibenclamide (n = 6), and placebo (n = 6) on insulin and leptin levels during 24-h periods before and after 16 weeks of therapy. RESULTS: We observed a significant diurnal variation in leptin concentrations. This was inversely related to insulin levels during the 24-h follow-up with usual diet. Neither the placebo nor acarbose altered leptin concentrations. However, glibenclamide increased leptin concentrations parallel to insulin levels. There were only minor changes in body weight during the l6-week follow-up: decrease in the placebo group (change -0.5 kg/m2, P = 0.07) and acarbose (change -0.7 kg/m2, P = 0.046) and increase in the glibenclamide group (change 0.8 kg/m2, P = 0.27). However, individual subjects who gained weight had increases in their leptin concentrations. The diurnal variation in leptin concentrations was preserved after glibenclamide. CONCLUSIONS: Glibenclamide increases circadian leptin and insulin concentrations, whereas acarbose does not. This observation may help to explain weight gain in subjects treated with glibenclamide and stable weight in those treated with acarbose in the long run.
Glibenclamide Prevents Hypoglycemia-Induced Fatal Cardiac Arrhythmias in Rats.[Pubmed:29800118]
Endocrinology. 2018 Jul 1;159(7):2614-2620.
Sulfonylureas increase the incidence of severe hypoglycemia in people with type 2 diabetes and might increase the risk of sudden cardiac death. Sulfonylureas stimulate insulin secretion by closing pancreatic ATP-sensitive potassium ion (KATP) channels. To investigate the role of KATP channel modulators on cardiac arrhythmias and mortality in the setting of severe hypoglycemia, adult Sprague-Dawley rats underwent hyperinsulinemic (0.2 U/kg/min) severe hypoglycemic (10 to 15 mg/dL) clamps with continuous electrocardiography. The rats were randomized for treatment with intravenous vehicle (VEH), the sulfonylurea glibenclamide (GLIB; KATP channel blocker; 5 mg/kg/h), or diazoxide (DIAZ; KATP channel opener; 5 mg/kg/h). The results demonstrated that GLIB completely prevented first-degree heart block compared with VEH (0.18 +/- 0.09/min) and DIAZ (0.2 +/- 0.05/min). Second-degree heart block was significantly reduced with GLIB (0.12 +/- 0.1/min) compared with VEH (0.6 +/- 0.2/min) and DIAZ (6.9 +/- 3/min). The incidence of third-degree heart block was completely prevented by GLIB compared with VEH (67%) and DIAZ (87.5%). Hypoglycemia-induced mortality was completely prevented by GLIB compared with VEH (60%) and DIAZ (82%). In conclusion, although GLIB increases the risk of hypoglycemia by increasing insulin secretion, these results have demonstrated a paradoxical protective role of GLIB against severe hypoglycemia-induced fatal cardiac arrhythmias.
Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents.[Pubmed:1281220]
J Gen Physiol. 1992 Oct;100(4):573-91.
The cystic fibrosis transmembrane conductance regulator (CFTR) is a Cl- channel that is regulated by cAMP-dependent phosphorylation and by intracellular ATP. Intracellular ATP also regulates a class of K+ channels that have a distinct pharmacology: they are inhibited by sulfonylureas and activated by a novel class of drugs called K+ channel openers. In search of modulators of CFTR Cl- channels, we examined the effect of sulfonylureas and K+ channel openers on CFTR Cl- currents in cells expressing recombinant CFTR. The sulfonylureas, tolbutamide and glibenclamide, inhibited whole-cell CFTR Cl- currents at half-maximal concentrations of approximately 150 and 20 microM, respectively. Inhibition by both agents showed little voltage dependence and developed slowly; > 90% inhibition occurred 3 min after adding 1 mM tolbutamide or 100 microM glibenclamide. The effect of tolbutamide was reversible, while that of glibenclamide was not. In contrast to their activating effect on K+ channels, the K+ channel openers, diazoxide, BRL 38227, and minoxidil sulfate inhibited CFTR Cl- currents. Half-maximal inhibition was observed at approximately 250 microM diazoxide, 50 microM BRL 38227, and 40 microM minoxidil sulfate. The rank order of potency for inhibition of CFTR Cl- currents was: glibenclamide < BRL 38227 approximately equal to minoxidil sulfate > tolbutamide > diazoxide. Site-directed mutations of CFTR in the first membrane-spanning domain and second nucleotide-binding domain did not affect glibenclamide inhibition of CFTR Cl- currents. However, when part of the R domain was deleted, glibenclamide inhibition showed significant voltage dependence. These agents, especially glibenclamide, which was the most potent, may be of value in identifying CFTR Cl- channels. They or related analogues might also prove to be of value in treating diseases such as diarrhea, which may involve increased activity of the CFTR Cl- channel.
Glipizide: a review of its pharmacological properties and therapeutic use.[Pubmed:389600]
Drugs. 1979 Nov;18(5):329-53.
Glipizide is a 'second generation' oral hypoglycaemic agent similar in potency to glibenclamide. It is completely absorbed after oral administration and has a rapid onset of action, but the duration of its hypoglycaemic effect is shorter than that of glibenclamide. It is rapidly metabolised to inactive metabolites which are excreted in the urine. Therapeutic trials have shown the efficacy of glipizide in maturity onset diabetes mellitus to be comparable with that of glibenclamide and chlorpropamide in newly diagnosed patients unresponsive to diet as well as in patients previously treated with oral hypoglycaemic drugs. Glipizide is well tolerated, but careful adjustment of dosage and attention to diet may be needed to avoid hypoglycaemic symptoms a few hours after a single daily dose.


