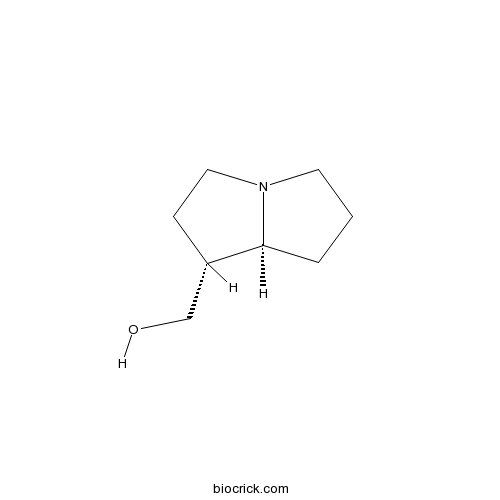
TrachelanthamidineCAS# 526-64-7 |
- Laburnine
Catalog No.:BCN1992
CAS No.:3348-73-0
- Isoretronecanol
Catalog No.:BCN1993
CAS No.:526-63-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 526-64-7 | SDF | Download SDF |
| PubChem ID | 188288 | Appearance | Oil |
| Formula | C8H15NO | M.Wt | 141.21 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R,8S)-2,3,5,6,7,8-hexahydro-1H-pyrrolizin-1-yl]methanol | ||
| SMILES | C1CC2C(CCN2C1)CO | ||
| Standard InChIKey | LOFDEIYZIAVXHE-YUMQZZPRSA-N | ||
| Standard InChI | InChI=1S/C8H15NO/c10-6-7-3-5-9-4-1-2-8(7)9/h7-8,10H,1-6H2/t7-,8-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Angew Chem Int Ed Engl. 2014 Nov 24;53(48):13196-200.Organocatalytic asymmetric Mannich cyclization of hydroxylactams with acetals: total syntheses of (-)-epilupinine, (-)-tashiromine, and (-)-trachelanthamidine.[Pubmed: 25264221]
J Org Chem. 2010 Jun 4;75(11):3578-86.Asymmetric synthesis of 2-alkyl-substituted 2,5-dihydropyrroles from optically active aza-Baylis-Hillman adducts. Formal synthesis of (-)-trachelanthamidine.[Pubmed: 20465267]A series of optically active 2-alkyl-substituted 2,5-dihydropyrroles were prepared via the asymmetric aza-Baylis-Hillman equivalent reaction and subsequent ring-closure metathesis reaction.
|

Trachelanthamidine Dilution Calculator

Trachelanthamidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.0817 mL | 35.4083 mL | 70.8165 mL | 141.633 mL | 177.0413 mL |
| 5 mM | 1.4163 mL | 7.0817 mL | 14.1633 mL | 28.3266 mL | 35.4083 mL |
| 10 mM | 0.7082 mL | 3.5408 mL | 7.0817 mL | 14.1633 mL | 17.7041 mL |
| 50 mM | 0.1416 mL | 0.7082 mL | 1.4163 mL | 2.8327 mL | 3.5408 mL |
| 100 mM | 0.0708 mL | 0.3541 mL | 0.7082 mL | 1.4163 mL | 1.7704 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isoretronecanol
Catalog No.:BCN1993
CAS No.:526-63-6
- Tryptophol
Catalog No.:BCN5681
CAS No.:526-55-6
- Abrine
Catalog No.:BCN2348
CAS No.:526-31-8
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Eudesmin
Catalog No.:BCN6563
CAS No.:526-06-7
- Iriflophenone
Catalog No.:BCN5679
CAS No.:52591-10-3
- Neophellamuretin
Catalog No.:BCN7888
CAS No.:52589-20-5
- Phellamurin
Catalog No.:BCN5678
CAS No.:52589-11-4
- Acm-thiopropionic acid
Catalog No.:BCC2838
CAS No.:52574-08-0
- Glycozolinine
Catalog No.:BCN5677
CAS No.:5257-08-9
- bPiDDB
Catalog No.:BCC7606
CAS No.:525596-66-1
- Ingenol 3-palmitate
Catalog No.:BCN7686
CAS No.:52557-26-3
- D(-)-Tartaric acid
Catalog No.:BCN8460
CAS No.:526-83-0
- Conduritol A
Catalog No.:BCN5683
CAS No.:526-87-4
- H-Sar-OEt.HCl
Catalog No.:BCC3334
CAS No.:52605-49-9
- Epipterosin L
Catalog No.:BCN5680
CAS No.:52611-75-3
- Deacetylasperulosidic acid methyl ester
Catalog No.:BCN1427
CAS No.:52613-28-2
- Ponicidin
Catalog No.:BCN3231
CAS No.:52617-37-5
- Isomorellic acid
Catalog No.:BCN3074
CAS No.:5262-69-1
- Gnetofuran B
Catalog No.:BCN7764
CAS No.:526214-79-9
- Huwentoxin IV
Catalog No.:BCC6270
CAS No.:526224-73-7
- Dehydroadynerigenin digitaloside
Catalog No.:BCN4623
CAS No.:52628-62-3
- Siegeskaurolic acid
Catalog No.:BCN6982
CAS No.:52645-97-3
- Anisodine
Catalog No.:BCN1868
CAS No.:52646-92-1
Asymmetric synthesis of 2-alkyl-substituted 2,5-dihydropyrroles from optically active aza-Baylis-Hillman adducts. Formal synthesis of (-)-trachelanthamidine.[Pubmed:20465267]
J Org Chem. 2010 Jun 4;75(11):3578-86.
A series of optically active 2-alkyl-substituted 2,5-dihydropyrroles were prepared via the asymmetric aza-Baylis-Hillman equivalent reaction and subsequent ring-closure metathesis reaction. Optically active aza-Baylis-Hillman adducts underwent a smooth two-step conversion to N-allyl-beta-amino-alpha-methylene esters in high yield, which gave chiral 2,5-dihydropyrroles, potential precursors for the aza-heterocyclic synthesis, almost quantitatively through RCM reaction catalyzed by Grubbs catalyst. The conversion was carried out without loss of the optical purity of the starting material. Synthetic application of the method to (-)-Trachelanthamidine was examined. Hydrogenation of 2,5-dihydropyrrole took place smoothly to give the corresponding 2,3-disubstituted pyrrolidine in good yield. The stereoselectivity of the hydrogenation was sensitive to the presence or absence of the protective group in the C2-side chain. The TBS-protected 2,5-dihydropyrrole gave a 1:1 mixture of the cis/trans isomers, while free alcohol afforded the trans-2,3-disubstituted pyrrolidine in a selectivity of 6:1. The formal synthesis of (-)-Trachelanthamidine was achieved in 11 steps from a chiral sulfinimine. This methodology provided a convenient procedure for the preparation of C2-alkyl-substituted 2,5-dihydropyroles with retention of high optical purity.
Organocatalytic asymmetric Mannich cyclization of hydroxylactams with acetals: total syntheses of (-)-epilupinine, (-)-tashiromine, and (-)-trachelanthamidine.[Pubmed:25264221]
Angew Chem Int Ed Engl. 2014 Nov 24;53(48):13196-200.
An asymmetric, organocatalytic, one-pot Mannich cyclization between a hydroxylactam and acetal is described to provide fused, bicyclic alkaloids bearing a bridgehead N atom. Both aliphatic and aromatic substrates were used in this transformation to furnish chiral pyrrolizidinone, indolizidinone, and quinolizidinone derivatives in up to 89% yield and 97% ee. The total syntheses of (-)-epilupinine, (-)-tashiromine, and (-)-Trachelanthamidine also achieved to demonstrate the generality of the process.


