TetrahydromagnololCAS# 20601-85-8 |
Quality Control & MSDS
Number of papers citing our products

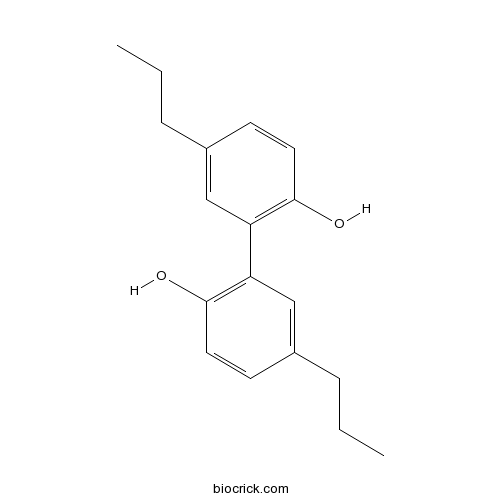
Chemical structure

3D structure
| Cas No. | 20601-85-8 | SDF | Download SDF |
| PubChem ID | 5321851 | Appearance | Powder |
| Formula | C18H22O2 | M.Wt | 270.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(2-hydroxy-5-propylphenyl)-4-propylphenol | ||
| SMILES | CCCC1=CC(=C(C=C1)O)C2=C(C=CC(=C2)CCC)O | ||
| Standard InChIKey | OYAQUBKYAKSHOA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H22O2/c1-3-5-13-7-9-17(19)15(11-13)16-12-14(6-4-2)8-10-18(16)20/h7-12,19-20H,3-6H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Tetrahydromagnolol can activate cannabinoid (CB) receptors. |
| Targets | Cannabinoid Receptor | GPR | GABA Receptor |

Tetrahydromagnolol Dilution Calculator

Tetrahydromagnolol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6982 mL | 18.4911 mL | 36.9822 mL | 73.9645 mL | 92.4556 mL |
| 5 mM | 0.7396 mL | 3.6982 mL | 7.3964 mL | 14.7929 mL | 18.4911 mL |
| 10 mM | 0.3698 mL | 1.8491 mL | 3.6982 mL | 7.3964 mL | 9.2456 mL |
| 50 mM | 0.074 mL | 0.3698 mL | 0.7396 mL | 1.4793 mL | 1.8491 mL |
| 100 mM | 0.037 mL | 0.1849 mL | 0.3698 mL | 0.7396 mL | 0.9246 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-D-Asn-OH.H2O
Catalog No.:BCC2879
CAS No.:2058-58-4
- Oxytetracycline hydrochloride
Catalog No.:BCC9110
CAS No.:2058-46-0
- Calycosin
Catalog No.:BCN5930
CAS No.:20575-57-9
- SB273005
Catalog No.:BCC6501
CAS No.:205678-31-5
- Orexin B (human)
Catalog No.:BCC5765
CAS No.:205640-91-1
- Orexin A (human, rat, mouse)
Catalog No.:BCC5764
CAS No.:205640-90-0
- alpha-Chaconine
Catalog No.:BCN2162
CAS No.:20562-03-2
- alpha-Solanine
Catalog No.:BCN2701
CAS No.:20562-02-1
- Oxibendazole
Catalog No.:BCC4818
CAS No.:20559-55-1
- Pterocarpadiol D
Catalog No.:BCN7760
CAS No.:2055882-23-8
- Pterocarpadiol C
Catalog No.:BCN7759
CAS No.:2055882-22-7
- Pterocarpadiol A
Catalog No.:BCN7758
CAS No.:2055882-21-6
- Ergosterol peroxide
Catalog No.:BCN4897
CAS No.:2061-64-5
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- CB30865
Catalog No.:BCC1457
CAS No.:206275-15-2
- Encecalin
Catalog No.:BCN4898
CAS No.:20628-09-5
- Calycosin-7-O-beta-D-glucoside
Catalog No.:BCN5931
CAS No.:20633-67-4
- Monomelittoside
Catalog No.:BCN8509
CAS No.:20633-72-1
- L-R4W2
Catalog No.:BCC5779
CAS No.:206350-79-0
- Darunavir
Catalog No.:BCC3623
CAS No.:206361-99-1
- Coniferaldehyde
Catalog No.:BCN4899
CAS No.:20649-42-7
- Sinapaldehyde
Catalog No.:BCN4900
CAS No.:20649-43-8
- Carmichaenine A
Catalog No.:BCN7729
CAS No.:2065228-59-1
- Carmichaenine B
Catalog No.:BCN7733
CAS No.:2065228-60-4
Magnolia Extract, Magnolol, and Metabolites: Activation of Cannabinoid CB2 Receptors and Blockade of the Related GPR55.[Pubmed:24900561]
ACS Med Chem Lett. 2012 Nov 14;4(1):41-5.
The bark of Magnolia officinalis is used in Asian traditional medicine for the treatment of anxiety, sleeping disorders, and allergic diseases. We found that the extract and its main bioactive constituents, magnolol and honokiol, can activate cannabinoid (CB) receptors. In cAMP accumulation studies, magnolol behaved as a partial agonist (EC50 = 3.28 muM) with selectivity for the CB2 subtype, while honokiol was less potent showing full agonistic activity at CB1 and antagonistic properties at CB2. We subsequently synthesized the major metabolites of magnolol and found that Tetrahydromagnolol (7) was 19-fold more potent than magnolol (EC50 CB2 = 0.170 muM) exhibiting high selectivity versus CB1. Additionally, 7 behaved as an antagonist at GPR55, a CB-related orphan receptor (K B = 13.3 muM, beta-arrestin translocation assay). Magnolol and its metabolites may contribute to the biological activities of Magnolia extract via the observed mechanisms of action. Furthermore, the biphenylic compound magnolol provides a simple novel lead structure for the development of agonists for CB receptors and antagonists for the related GPR55.
Is there a potential of misuse for Magnolia officinalis compounds/metabolites?[Pubmed:28517911]
Hum Psychopharmacol. 2017 May;32(3).
OBJECTIVE: Magnolia bark contains magnolol, metabolized to Tetrahydromagnolol and honokiol, with both GABA-ergic/cannabimimetic activities, hence of possible attraction to vulnerable individuals/recreational misusers. METHODS: A literature review, assessment of related anecdotal online Magnolia misuse's reports and an overview of Magnolia products' online acquisition possibilities has been here described. RESULTS: No peer-reviewed papers about Magnolia abuse/misuse/dependence/addiction were identified. Conversely, from a range of websites emerged potentially 3 groups of Magnolia misusers: (a) subjects with a psychiatric history already treated with benzodiazepines, being attracted to Magnolia bark as a "natural sedative"; (b) polydrug misusers, ingesting Magnolia with a range of other herbs/plants, attracted by the GABA-ergic/cannabimimetic activities; (c) subjects naive to the misusing drugs' scenario, perceiving Magnolia as a natural dietary supplement/weight-control compound. CONCLUSIONS: To the best of our knowledge, this is the first paper commenting on the possible Magnolia derivatives' potential of misuse. Magnolia's recent increase in popularity, mainly as a sedative, may be arguably due to its peculiar pharmacological properties/acceptable affordability levels/virtually worldwide favorable legal status and customers' attraction to a product being perceived as "natural" and hence somehow "safe." Future/potent/synthetic magnolol and honokiol structural analogues could however contribute to increasing the number of synthetic GABA-ergic/cannabimimetic misusing compounds.
The natural product magnolol as a lead structure for the development of potent cannabinoid receptor agonists.[Pubmed:24204944]
PLoS One. 2013 Oct 30;8(10):e77739.
Magnolol (4-allyl-2-(5-allyl-2-hydroxyphenyl)phenol), the main bioactive constituent of the medicinal plant Magnolia officinalis, and its main metabolite Tetrahydromagnolol were recently found to activate cannabinoid (CB) receptors. We now investigated the structure-activity relationships of (tetrahydro)magnolol analogs with variations of the alkyl chains and the phenolic groups and could considerably improve potency. Among the most potent compounds were the dual CB1/CB2 full agonist 2-(2-methoxy-5-propyl-phenyl)-4-hexylphenol (61a, K(i) CB1:0.00957 microM; K(i) CB2:0.0238 microM), and the CB2-selective partial agonist 2-(2-hydroxy-5-propylphenyl)-4-pentylphenol (60, K(i) CB1:0.362 microM; K(i ) CB2:0.0371 microM), which showed high selectivity versus GPR18 and GPR55. Compound 61b, an isomer of 61a, was the most potent GPR55 antagonist with an IC50 value of 3.25 microM but was non-selective. The relatively simple structures, which possess no stereocenters, are easily accessible in a four- to five-step synthetic procedure from common starting materials. The central reaction step is the well-elaborated Suzuki-Miyaura cross-coupling reaction, which is suitable for a combinatorial chemistry approach. The scaffold is versatile and may be fine-tuned to obtain a broad range of receptor affinities, selectivities and efficacies.


