RhamnazinCAS# 552-54-5 |
Quality Control & MSDS
Number of papers citing our products

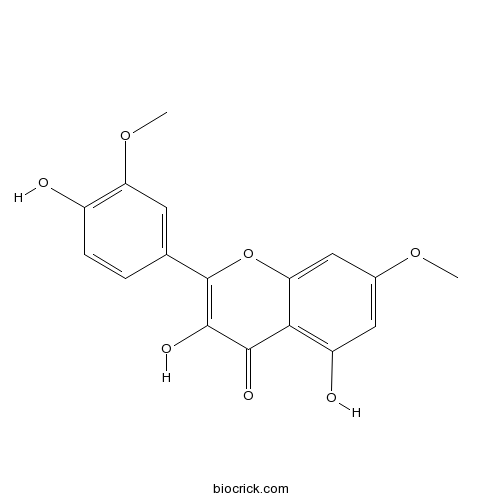
Chemical structure

3D structure
| Cas No. | 552-54-5 | SDF | Download SDF |
| PubChem ID | 5320945 | Appearance | Yellow powder |
| Formula | C17H14O7 | M.Wt | 330.29 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,5-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-7-methoxychromen-4-one | ||
| SMILES | COC1=CC(=C2C(=C1)OC(=C(C2=O)O)C3=CC(=C(C=C3)O)OC)O | ||
| Standard InChIKey | MYMGKIQXYXSRIJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O7/c1-22-9-6-11(19)14-13(7-9)24-17(16(21)15(14)20)8-3-4-10(18)12(5-8)23-2/h3-7,18-19,21H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Rhamnazin Dilution Calculator

Rhamnazin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0276 mL | 15.1382 mL | 30.2764 mL | 60.5528 mL | 75.6911 mL |
| 5 mM | 0.6055 mL | 3.0276 mL | 6.0553 mL | 12.1106 mL | 15.1382 mL |
| 10 mM | 0.3028 mL | 1.5138 mL | 3.0276 mL | 6.0553 mL | 7.5691 mL |
| 50 mM | 0.0606 mL | 0.3028 mL | 0.6055 mL | 1.2111 mL | 1.5138 mL |
| 100 mM | 0.0303 mL | 0.1514 mL | 0.3028 mL | 0.6055 mL | 0.7569 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sonnerphenolic B
Catalog No.:BCN9232
CAS No.:1627516-10-2
- Axinysone A
Catalog No.:BCN9231
CAS No.:1114491-57-4
- N-Acetyldelectine
Catalog No.:BCN9230
CAS No.:63596-61-2
- (-)-Episyringaresinol
Catalog No.:BCN9229
CAS No.:6216-82-6
- Glabredelphinine
Catalog No.:BCN9228
CAS No.:132160-37-3
- Delbonine
Catalog No.:BCN9227
CAS No.:95066-33-4
- Sepiumol A
Catalog No.:BCN9226
CAS No.:2411999-52-3
- (+)-Nyasol
Catalog No.:BCN9225
CAS No.:185020-38-6
- 5,7-Dihydroxyphthalide
Catalog No.:BCN9224
CAS No.:27979-58-4
- Desglucohellebrin
Catalog No.:BCN9223
CAS No.:20300-44-1
- 6,4'-Dihydroxy-7-methoxyflavan
Catalog No.:BCN9222
CAS No.:202463-50-1
- Ajacine
Catalog No.:BCN9221
CAS No.:509-17-1
- 4-Hydroxy-2,3-dimethoxyxanthone
Catalog No.:BCN9234
CAS No.:10527-38-5
- 2-(2,4-Dihydroxybenzoyl)benzoic acid
Catalog No.:BCN9235
CAS No.:2513-33-9
- (R)-4-Methoxydalbergione
Catalog No.:BCN9236
CAS No.:4646-86-0
- Qingyangshengenin 3-O-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-digitoxopyranoside
Catalog No.:BCN9237
CAS No.:1186628-88-5
- Carpinontriol A
Catalog No.:BCN9238
CAS No.:473451-72-8
- Alnusonol
Catalog No.:BCN9239
CAS No.:52330-12-8
- Sepiumol C
Catalog No.:BCN9240
CAS No.:2412138-09-9
- Periplanetin
Catalog No.:BCN9241
CAS No.:21056-52-0
- Otophylloside T
Catalog No.:BCN9242
CAS No.:1642306-14-6
- Cinchonain Ia
Catalog No.:BCN9243
CAS No.:85081-24-9
- Lehmbachol D
Catalog No.:BCN9244
CAS No.:913556-40-8
- Dehydrojuncuenin A
Catalog No.:BCN9245
CAS No.:1161681-26-0
Unmodified household coffee maker assisted extraction and purification of anticancer agents from Dillenia indica fruits.[Pubmed:31134812]
Nat Prod Res. 2019 May 28:1-4.
Bioassay targeted, 80% aqueous ethanol crude extract of the fruits of Dillenia indica Linn, using the unmodified household coffee maker, afforded five compounds, namely betulinic acid (1), Rhamnazin (2), dillenetin (3), luteolin-7-O-beta-D-glucoside (4) and hypolaetin-8-O-beta-D-glucoside (5). The crude extract, fractions and purified compounds were tested against MDA MB-231, A549 and HeLa cancer cell lines by MTT assay, using betulinic acid 1, as a positive control. Compound 3 showed the best activity against A549 (IC50 = 26.60 +/- 2.5 microM) and HeLa cancer cell lines (IC50 =19.35 +/- 0.9 microM), whereas compound 5 was found to show the best activity against MDA MB-231 (IC50 = 34.62 +/- 5.2microM) cancer cell line. These highly potent anticancer compounds obtained from the fruits of D. indica may be suitable for herbal drug development and formulations.
In vitro inhibition of Hepatitis C virus protease and antioxidant by flavonoid glycosides from the Saudi costal plant Sarcocornia fruticosa.[Pubmed:29897265]
Nat Prod Res. 2019 Dec;33(23):3364-3371.
A new flavonol triglycoside, Rhamnazin 3-O-2(G)-rhamnorutinoside or Rhamnazin 3-O-(2'',6''-O-alpha-di-rhamnosyl)-beta-glucoside (1) was isolated along with known flavonols, Rhamnazin 3-O-rutinoside (2), Rhamnazin 3-O-(6''-O-alpha-rhamnosyl)-beta-galactoside (3), isorhamnetin 3-O-(6''-O-alpha-rhamnosyl)-beta-galactoside (4), isorhamnetin 3-O-(2'',6''-O-alpha-di-rhamnosyl)-beta-galactoside (5), and isorhamnetin (6), and allantoin (7) from the aqueous methanol extract of Sarcocornia fruticosa leaves. Spectral analyses (UV, MS, and NMR) and acid hydrolysis were used to determine the structures. These compounds in this study except 6 were reported for the first time from the genus Sarcocornia. The extract and flavonol glycosides (1-5) were evaluated for antioxidant and inhibition of HCV protease enzyme. Rhamnazin triglycoside (1) was shown to have a potent HCV protease inhibitor with IC50 value 8.9 muM, while isorhamnetin di- and triglycosides (4 and 5) were effectively scavenged DPPH radicals with IC50 values 3.8 and 4.3 muM, respectively.
Crispoic acid, a new compound from Laelia marginata (Orchidaceae), and biological evaluations against parasites, human cancer cell lines and Zika virus.[Pubmed:29117727]
Nat Prod Res. 2018 Dec;32(24):2916-2921.
The phytochemical study of Laelia marginata (Lindl.) L. O. Williams (Orchidaceae) led to the isolation of a new natural product named crispoic acid (1), together with six other known compounds (2-7). The new natural product was identified as a dimer of eucomic acid and was structurally characterised based upon 1D and 2D NMR and HRMS data. Biological assays with plant crude extract, fractions and isolated compounds were performed against two human cancer cell lines (Hela and Siha), and the tropical parasites Trypanosoma cruzi and Leishmania (Leishmania) amazonensis. The phenantrenoid 9,10-dihydro-4-methoxyphenanthren-2,7-diol 2 was active against Hela and Siha cells (CC50 5.86 +/- 0.19 and 20.78 +/- 2.72 mug/mL, respectively). Sub-lethal concentrations of the flavone Rhamnazin 4 were not able to rescue the viability of the Vero cells infected by Zika virus.
Evaluation of Rhamnetin as an Inhibitor of the Pharmacological Effect of Secretory Phospholipase A2.[Pubmed:28858248]
Molecules. 2017 Aug 31;22(9). pii: molecules22091441.
Rhamnetin (Rhm), 3-O-methylquercetin (3MQ), and Rhamnazin (Rhz) are methylated derivatives of quercetin commonly found in fruits and vegetables that possess antioxidant and anti-inflammatory properties. Phospholipase A2 (PLA2) displays several important roles during acute inflammation; therefore, this study aimed at investigating new compounds able to inhibit this enzyme, besides evaluating creatine kinase (CK) levels and citotoxicity. Methylated quercetins were compared with quercetin (Q) and were incubated with secretory PLA2 (sPLA2) from Bothrops jararacussu to determine their inhibitory activity. Cytotoxic studies were performed by using the J774 cell lineage incubated with quercertins. In vivo tests were performed with Swiss female mice to evaluate decreasing paw edema potential and compounds' CK levels. Structural modifications on sPLA2 were made with circular dichroism (CD). Despite Q and Rhz showing greater enzymatic inhibitory potential, high CK was observed. Rhm exhibited sPLA2 inhibitory potential, no toxicity and, remarkably, it decreased CK levels. The presence of 3OH on the C-ring of Rhm may contribute to both its anti-inflammatory and enzymatic inhibition of sPLA2, and the methylation of ring A may provide the increase in cell viability and low CK level induced by sPLA2. These results showed that Rhm can be a candidate as a natural compound for the development of new anti-inflammatory drugs.
Two new linear acetogenins from the fruits of Goniothalamus gracilipes.[Pubmed:28714315]
Nat Prod Res. 2018 Feb;32(3):287-293.
Two new linear acetogenins, gracilipin A (1) and methylsaccopetrin A (2) along with seven known compounds, saccopetrin A (3), 7,3',4'-trimethylquercetin (4), Rhamnazin (5), casticin (6), isokanugin (7), melisimplexin (8) and 5-hydroxy-3,7-dimethoxy-3',4'-methylenedioxyflavone (9) were isolated from the fruits of Goniothalamus gracilipes Ban. Their structures were established by spectral analysis, such as mass spectrometry, 1D-NMR, 2D-NMR and circular dichroism (CD). Compounds 1 and 3 showed cytotoxic activity against KB cell line with IC50 values of 14.6 and 15.3 muM, respectively.
ANTIOXIDANT AND ANTI-INFLAMMATORY EFFECTS OF RHAMNAZIN ON LIPOPOLYSACCHARIDE-INDUCED ACUTE LUNG INJURY AND INFLAMMATION IN RATS.[Pubmed:28638883]
Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4):201-212.
BACKGROUND: Acute Lung Injury (ALI) results into severe inflammation and oxidative stress to the pulmonary tissue. Rhamnazin is a natural flavonoid and known for its antioxidant and anti-inflammatory properties. MATERIALS AND METHODS: The antioxidative and anti-inflammatory properties Rhamnazin were tested for protection against the acute lung injury. We investigated whether Rhamnazin improves the lipopolysaccharide (LPS)-induced ALI in an animal model (rat). We also studied the probable molecular mechanism of action of Rhamnazin. Rhamnazin was injected intraperitoneally (i.p.) (5, 10 and 20 mg/kg) two days before intratracheal LPS challenge (5mg/kg). The changes in lung wet-to-dry weight ratio, LDH activity, pulmonary histopathology, BALF protein concentration, MPO activity, oxidative stress, cytokine production were estimated. RESULTS: The results showed a significant attenuation of all the inflammatory parameters and a marked improvement in the pulmonary histopathology in the animal groups pretreated with Rhamnazin. The Rhamnazin pretreated group also showed activation of Nrf2 pathway and attenuation of ROS such as H2O2, MDA and hydroxyl ion. These results indicated that Rhamnazin could attenuate the symptoms of ALI in rats due to its strong antioxidant and anti-inflammatory properties. CONCLUSION: The results strongly demonstrated that Rhamnazin provides protection against LPS-induced ALI. The underlying mechanisms of its anti-inflammatory action may include inhibition of Nrf2 mediated antioxidative pathway.
Biotechnological Production of Dimethoxyflavonoids Using a Fusion Flavonoid O-Methyltransferase Possessing Both 3'- and 7-O-Methyltransferase Activities.[Pubmed:28429944]
J Nat Prod. 2017 May 26;80(5):1467-1474.
Although they are less abundant in nature, methoxyflavonoids have distinct physicochemical and pharmacological properties compared to common nonmethylated flavonoids. Thus, enzymatic conversion and biotransformation using genetically engineered microorganisms of flavonoids have been attempted for the efficient production of methoxyflavonoids. Because of their regiospecificity, more than two flavonoid O-methyltransferases (FOMTs) and enzyme reactions are required to biosynthesize di(or poly)-methoxyflavonoids. For the one-step biotechnological production of bioactive di-O-methylflavonoids, we generated a multifunctional FOMT fusing a 3'-OMT (SlOMT3) and a 7-OMT (OsNOMT). The SlOMT3/OsNOMT fusion enzyme possessed both 3'- and 7-OMT activities to diverse flavonoid substrates, which were comparable to those of individual SlOMT3 and OsNOMT. The SlOMT3/OsNOMT enzyme also showed 3'- and 7-OMT activity for 7- or 3'-O-methylflavonoids, respectively, suggesting that the fusion enzyme can sequentially methylate flavonoids into di-O-methylflavonoids. The biotransformation of the flavonoids quercetin, luteolin, eriodictyol, and taxifolin using SlOMT3/OsNOMT-transformed Escherichia coli generated corresponding di-O-methylflavonoids, Rhamnazin, velutin, 3',7-di-O-methyleriodictyol, and 3',7-di-O-methyltaxifolin, respectively. These results indicate that dimethoxyflavonoids may be efficiently produced from nonmethylated flavonoid precursors through a one-step biotransformation using the engineered E. coli harboring the SlOMT3/OsNOMT fusion gene.
Effect of O-methylated and glucuronosylated flavonoids from Tamarix gallica on alpha-glucosidase inhibitory activity: structure-activity relationship and synergistic potential.[Pubmed:27838961]
Biosci Biotechnol Biochem. 2017 Mar;81(3):445-448.
O-Methylated and glucuronosylated flavonoids were isolated from Tamarix gallica as alpha-glucosidase inhibitors. Structure-activity relationship of these flavonoids suggests that catechol moiety and glucuronic acid at C-3 are factors in the increase in alpha-glucosidase inhibitory activity. Furthermore, rhamnetin, tamarixetin, Rhamnazin, KGlcA, KGlcA-Me, QGlcA, and QGlcA-Me exhibit synergistic potential when applied with a very low concentration of acarbose to alpha-glucosidase from rat intestine.
Atmospheric solids analysis probe mass spectrometry for the rapid identification of pollens and semi-quantification of flavonoid fingerprints.[Pubmed:27321852]
Rapid Commun Mass Spectrom. 2016 Jul 15;30(13):1639-46.
RATIONALE: From allergies to plant reproduction, pollens have important impacts on the health of human and plant populations, yet identification of pollen grains remains difficult and time-consuming. Low-volatility flavonoids generated from pollens cannot be easily characterized and quantified with current analytical techniques. METHODS: Here we present the novel use of atmospheric solids analysis probe mass spectrometry (ASAP-MS) for the characterization of flavonoids in pollens. Flavonoid patterns were generated for pollens collected from different plant types (trees and bushes) in addition to bee pollens from distinct geographic regions. Standard flavonoids (kaempferol and Rhamnazin) and those produced from pollens were compared and assessed with ASAP-MS using low-energy collision MS/MS. Results for a semi-quantitative method for assessing the amount of a flavonoid in pollens are also presented. RESULTS: Flavonoid patterns for pollen samples were distinct with variability in the number and relative abundance of flavonoids in each sample. Pollens contained 2-5 flavonoids, and all but Kochia scoparia contained kaempferol or kaempferol isomers. We establish this method as a reliable and applicable technique for analyzing low-volatility compounds with minimal sample preparation. Standard curves were generated using 0.2-5 mug of kaempferol; from these experiments, it was estimated that there is approximately 2 mg of kaempferol present in 1 g of P. nigra italica pollen. CONCLUSIONS: Pollens can be characterized with a simple flavonoid pattern rather than analyzing the whole product pattern or the products-temperature profiles. ASAP-MS is a rapid analytical technique that can be used to distinguish between plant pollens and between bee pollens originating from different regions. Copyright (c) 2016 John Wiley & Sons, Ltd.
New Flavonol Glucuronides from the Flower Buds of Syzygium aromaticum (Clove).[Pubmed:27045836]
J Agric Food Chem. 2016 Apr 20;64(15):3048-53.
Repeated chromatography of the EtOAc-soluble fraction from the 70% EtOH extract of the flower buds of Syzygium aromaticum (clove) led to the isolation and characterization of four new flavonol glucuronides, rhamnetin-3-O-beta-d-glucuronide (1), Rhamnazin-3-O-beta-d-glucuronide (2), Rhamnazin-3-O-beta-d-glucuronide-6''-methyl ester (3), and rhamnocitrin-3-O-beta-d-glucuronide-6''-methyl ester (4), together with 15 flavonoids (5-19) having previously known chemical structures. The structures of the new compounds 1-4 were determined by interpretation of spectroscopic data, particularly by 1D- and 2D-NMR studies. Six flavonoids (6, 7, 9, 14, 18, and 19) were isolated from the flower buds of S. aromaticum for the first time in this study. The flavonoids were examined for their cytotoxicity against human ovarian cancer cells (A2780) using MTT assays. Among the isolates, pachypodol (19) showed the most potent cytotoxicity on A2780 cells with an IC50 value of 8.02 muM.
RHAMNAZIN INHIBITS PROLIFERATION AND INDUCES APOPTOSIS OF HUMAN JURKAT LEUKEMIA CELLS IN VITRO.[Pubmed:27025066]
Ukr Biochem J. 2015 Nov-Dec;87(6):122-8.
Antiproliferative and apoptogenic effects of Rhamnazin, a dimethoxylated derivative of quercetin, were studied in human acute lymphoblastic leukemia Jurkat cells. The cytotoxicity and apoptogenic activity of Rhamnazin in vitro are inferior to that of quercetin. The apoptogenic activity of Rhamnazin is realized via mitochondrial pathway and associated with activation of caspase-9 and -3. The additive apoptogenic effect of Rhamnazin and suboptimal doses of etoposide, a DNA topoisomerase II inhibitor, is demonstrated. Therefore, methylation of quercetin modifies its biological effects considerably.
[Studies on flavonoids from stems of Nelumbo nucifera Gaertn and their cytotoxic activities].[Pubmed:25850268]
Zhongguo Zhong Yao Za Zhi. 2014 Nov;39(22):4360-4.
This research is to investigate study the flavonoids from stems of Nelumbo nucifera and the cytotoxic activities of iso- lated compounds. The constituents were separated by column chromatography,and their structures were elucidated by spectroscopic data analyses. The isolated compounds were evaluated for cytoxic activities by MTT method. Twelve compounds were isolated and identified as Rhamnazin-3-O-beta-D-glucopyranoside (1), luteolin-3', 4'-dimethylether-7-O-beta-D-glucoside (2), kaempferol-3-O-beta-D-xylopyranosyl-(1-->2)-O-beta-D-glucopyranoside (3), quercetin-3,3'-di-O-beta-D-glucopyranoside (4), 1, 8-dihydroxy-3,7-dimethoxyxanthone (5), isorhamnetin-3-O-beta-D-glucopyranoside(6) , kaempferol(7), isorhamnetin (8), quercetin(9), astragalin(10), hyperoside (11) and 1-hy- droxy-3,7,8-trimethoxyxanthone(12). All compounds were isolated from stems of this plant for the first time, and compounds 1-5 were firstly isolated from the family nelumbonaceae. Compounds 24 and 6 showed significant cytotoxic activities against BEL-7402 carcinoma cell lines at a concentration of 1 x 10(-5) mol x L(-1) with the inhibitory rate of 67.36%, 53.25%, 57.78%, 60.13% and 52.11%, respectively.
Rhamnazin, a novel inhibitor of VEGFR2 signaling with potent antiangiogenic activity and antitumor efficacy.[Pubmed:25704088]
Biochem Biophys Res Commun. 2015 Mar 20;458(4):913-9.
Anti-angiogenesis targeting vascular endothelial growth factor receptor 2 (VEGFR2) has emerged as an important tool for cancer therapy. The identification of new drugs from natural products has a long and successful history. In this study, we described a novel VEGFR2 inhibitor, Rhamnazin, which inhibits tumor angiogenesis and growth. Rhamnazin significantly inhibited proliferation, migration and tube formation of human umbilical vascular endothelial cells (HUVECs) in vitro as well as inhibited sprouts formation of rat aorta ring. In addition, it inhibited vascular endothelial growth factor (VEGF)-induced phosphorylation of VEGFR2 and its downstream signaling regulator in HUVECs. Moreover, Rhamnazin could directly inhibit proliferation of breast cancer cells MDA-MB-231 in vitro and in vivo. Oral administration of Rhamnazin at a dose of 200 mg/kg/day could markedly inhibited human tumor xenograft growth and decreased microvessel densities (MVD) in tumor sections. Taken together, these preclinical evaluations suggest that Rhamnazin inhibits angiogenesis and may be a promising anticancer drug candidate.
A new proline-containing flavonol glycoside from Caragana leucophloea Pojark.[Pubmed:25675255]
Nat Prod Res. 2015;29(19):1811-9.
One new proline-containing flavonol glycoside, namely kaempferol-3-O-methyl-7-O-beta-D-glucopyranosyl-8-(1-methyleneproline)-4'-O-beta- D-glucopyranoside (1), together with 15 known flavonoids, 3-O-methylkaempferol (2), 3-O-methylquercetin (3), quercetin (4), kaempferol (5), apigenin (6), Rhamnazin (7), astragalin (8), alquds (9), quercitrin (10), rutin (11), isoquercitrin (12), apigetrin (13), myricitrin (14), hesperidin (15) and calycosin-7-O-beta-D-glucopyranoside (16) were isolated from the aerial parts of Caragana leucophloea Pojark. (Leguminosae). Their structures were determined on the basis of spectroscopic analyses and by comparison with literature data. Compounds 2-4 revealed a strong antimicrobial activity with minimum inhibitory concentration values of 12.5-150 mug/mL and median inhibitory concentration (IC50) values of 7.42-76.61 mug/mL. Compounds 3, 4, 6-8, 10-12 and 14 showed strong antioxidant activity. Compounds 2-7 exhibited moderate antinematodal activity on Caenorhabditis elegans with IC50 values of 40.51-68.05 mug/mL.


