ProtopanaxatriolCAS# 32773-56-1 |
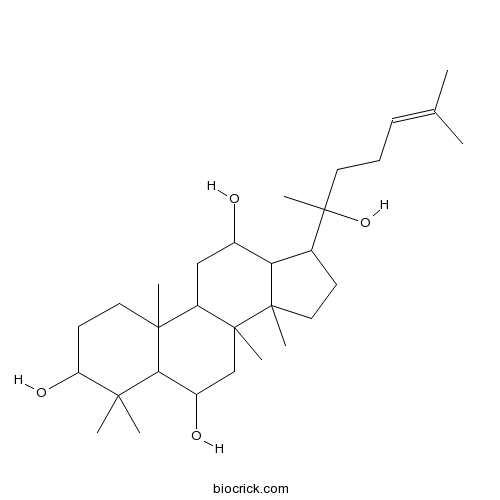
- 20(R)-Protopanaxatriol
Catalog No.:BCN1079
CAS No.:1453-93-6
- (20S)-Protopanaxatriol
Catalog No.:BCN2705
CAS No.:34080-08-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 32773-56-1 | SDF | Download SDF |
| PubChem ID | 22392424 | Appearance | Powder |
| Formula | C30H52O4 | M.Wt | 476.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 17-(2-hydroxy-6-methylhept-5-en-2-yl)-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene-3,6,12-triol | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CC(C4C3(CCC(C4(C)C)O)C)O)C)O)C)O)C | ||
| Standard InChIKey | SHCBCKBYTHZQGZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H52O4/c1-18(2)10-9-13-30(8,34)19-11-15-28(6)24(19)20(31)16-22-27(5)14-12-23(33)26(3,4)25(27)21(32)17-29(22,28)7/h10,19-25,31-34H,9,11-17H2,1-8H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Protopanaxatriol Dilution Calculator

Protopanaxatriol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0978 mL | 10.4888 mL | 20.9776 mL | 41.9551 mL | 52.4439 mL |
| 5 mM | 0.4196 mL | 2.0978 mL | 4.1955 mL | 8.391 mL | 10.4888 mL |
| 10 mM | 0.2098 mL | 1.0489 mL | 2.0978 mL | 4.1955 mL | 5.2444 mL |
| 50 mM | 0.042 mL | 0.2098 mL | 0.4196 mL | 0.8391 mL | 1.0489 mL |
| 100 mM | 0.021 mL | 0.1049 mL | 0.2098 mL | 0.4196 mL | 0.5244 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Macrocarpal O
Catalog No.:BCN7371
CAS No.:327622-65-1
- BIO 5192
Catalog No.:BCC8002
CAS No.:327613-57-0
- Macrocarpal L
Catalog No.:BCN5248
CAS No.:327601-97-8
- Phorbol 13-acetate
Catalog No.:BCN7231
CAS No.:32752-29-7
- Heliosupine
Catalog No.:BCN1980
CAS No.:32728-78-2
- tcY-NH2
Catalog No.:BCC5770
CAS No.:327177-34-4
- Cyclohexanecarboxylic acid
Catalog No.:BCN3443
CAS No.:98-89-5
- VU 0285683
Catalog No.:BCC6154
CAS No.:327056-22-4
- TDZD-8
Catalog No.:BCC4258
CAS No.:327036-89-5
- TCS-PIM-1-4a
Catalog No.:BCC5461
CAS No.:327033-36-3
- Chlorogenic acid
Catalog No.:BCN5906
CAS No.:327-97-9
- H-Nle-OH
Catalog No.:BCC3295
CAS No.:327-57-1
- Labetalol HCl
Catalog No.:BCC5489
CAS No.:32780-64-6
- Panaxatriol
Catalog No.:BCN1081
CAS No.:32791-84-7
- Nomifensine
Catalog No.:BCC7226
CAS No.:32795-47-4
- H-D-Leu-OH
Catalog No.:BCC2975
CAS No.:328-38-1
- Ceranib 1
Catalog No.:BCC6186
CAS No.:328076-61-5
- Phortress
Catalog No.:BCC3901
CAS No.:328087-38-3
- Coniferyl alcohol
Catalog No.:BCN4651
CAS No.:32811-40-8
- (H-Cys-OMe)2.2HCl
Catalog No.:BCC2916
CAS No.:32854-09-4
- Lannaconitine
Catalog No.:BCN2504
CAS No.:32854-75-4
- GlyH-101
Catalog No.:BCC4104
CAS No.:328541-79-3
- AG-14361
Catalog No.:BCC2209
CAS No.:328543-09-5
- Benzylamine hydrochloride
Catalog No.:BCN6908
CAS No.:3287-99-8
Co-administration of 20(S)-protopanaxatriol (g-PPT) and EGFR-TKI overcomes EGFR-TKI resistance by decreasing SCD1 induced lipid accumulation in non-small cell lung cancer.[Pubmed:30876460]
J Exp Clin Cancer Res. 2019 Mar 15;38(1):129.
BACKGROUND: Non-small cell lung cancer (NSCLC) patients with sensitive epidermal growth factor receptor (EGFR) mutations are successfully treated with EGFR tyrosine kinase inhibitors (EGFR-TKIs); however, resistance to treatment inevitably occurs. Given lipid metabolic reprogramming is widely known as a hallmark of cancer and intimately linked with EGFR-stimulated cancer growth. Activation of EGFR signal pathway increased monounsaturated fatty acids (MUFA) and lipid metabolism key enzyme Stearoyl-CoA Desaturase 1 (SCD1) expression. However the correlation between EGFR-TKI resistance and lipid metabolism remains to be determined. METHODS: In this study the differences in lipid synthesis between paired TKI-sensitive and TKI-resistant patient tissues and NSCLC cell lines were explored. Oleic acid (OA, a kind of MUFA, the SCD1 enzymatic product) was used to simulate a high lipid metabolic environment and detected the affection on the cytotoxic effect of TKIs (Gefitinib and osimertinib) in cell lines with EGFR-activating mutations. (20S)-Protopanaxatriol (g-PPT), an aglycone of ginsenosides, has been reported to be an effective lipid metabolism inhibitor, was used to inhibit lipid metabolism. Additionally, synergism in cytotoxic effects and signal pathway activation were evaluated using CCK-8 assays, Western blotting, flow cytometry, Edu assays, plate clone formation assays and immunofluorescence. Furthermore, two xenograft mouse models were used to verify the in vitro results. RESULTS: Gefitinib-resistant cells have higher lipid droplet content and SCD1 expression than Gefitinib-sensitive cells in both NSCLC cell lines and patient tissues. Additionally oleic acid (OA, a kind of MUFA, the SCD1 enzymatic product) abrogates the cytotoxic effect of both Gefitinib and osimertinib in cell lines with EGFR-activating mutations. As a reported effective lipid metabolism inhibitor, g-PPT significantly inhibited the expression of SCD1 in lung adenocarcinoma cells, and then down-regulated the content of intracellular lipid droplets. Combined treatment with Gefitinib and g-PPT reverses the resistance to Gefitinib and inhibits the activation of p-EGFR and the downstream signaling pathways. CONCLUSIONS: Our findings uncover a link between lipid metabolic reprogramming and EGFR-TKI resistance, confirmed that combination target both EGFR and abnormal lipid metabolism maybe a promising therapy for EGFR-TKI resistance and highlighting the possibility of monitoring lipid accumulation in tumors for predicting drug resistance.
Neuroprotective Effects of Dammarane-Type Saponins from Panax notoginseng on Glutamate-Induced Cell Damage in PC12 Cells.[Pubmed:30791058]
Planta Med. 2019 Feb 21.
Dammarane-type saponins, the main active ingredients of Panax notoginseng, have substantial neuroprotective effects in different animal models of neurodegenerative diseases. However, because these compounds have different structures, the level of protection provided by individual compounds varies, and highly active compounds can be selected based on structure-activity relationships. Glutamate is a major excitatory neurotransmitter that plays an important role in synaptic response development. However, excessive extracellular glutamate levels lead to neuronal dysfunctions in the central nervous system. Herein, we investigated the neuroprotective effects of nine saponins (compounds 1: - 9: ) on glutamate-treated PC12 cells in the concentration range of 0.1 - 10 microM. The MTT assay revealed that these compounds increased cell viability to 65.6, 69.8, 76.9, 91.7, 74.4, 63.3, 59.9, 64.7, and 59.9%, respectively, compared with the glutamate-treated cells (44.6%). Protopanaxatriol (compound 4: ) was the most neuroprotective compound, and subsequent experiments revealed that pretreatment with compound 4: significantly reverses mitochondrial membrane potential collapse, increases superoxide dismutase activity, and decreases lactate dehydrogenase leakage, malondiadehyde levels, reactive oxygen species generation, and cell apoptosis. Compound 4: also decreased the Bax/Bcl-2 ratio, cleaved caspase-3, N-methyl-D-aspartic receptor 1, and Ca(2+)-/calmodulin-dependent protein kinase II expression, and inhibited glutamate-induced cytochrome C release and phosphorylation of apoptosis signal-regulating kinase 1, c-Jun N-terminal kinase, and p38. Overall, the results indicate that Protopanaxatriol has significant neuroprotective effects, and might be a promising neuroprotective agent for preventing and treating neurodegenerative diseases.
Potential Dissociative Glucocorticoid Receptor Activity for Protopanaxadiol and Protopanaxatriol.[Pubmed:30591629]
Int J Mol Sci. 2018 Dec 27;20(1). pii: ijms20010094.
Glucocorticoids are steroid hormones that regulate inflammation, growth, metabolism, and apoptosis via their cognate receptor, the glucocorticoid receptor (GR). GR, acting mainly as a transcription factor, activates or represses the expression of a large number of target genes, among them, many genes of anti-inflammatory and pro-inflammatory molecules, respectively. Transrepression activity of glucocorticoids also accounts for their anti-inflammatory activity, rendering them the most widely prescribed drug in medicine. However, chronic and high-dose use of glucocorticoids is accompanied with many undesirable side effects, attributed predominantly to GR transactivation activity. Thus, there is a high need for selective GR agonist, capable of dissociating transrepression from transactivation activity. Protopanaxadiol and Protopanaxatriol are triterpenoids that share structural and functional similarities with glucocorticoids. The molecular mechanism of their actions is unclear. In this study applying induced-fit docking analysis, luciferase assay, immunofluorescence, and Western blot analysis, we showed that protopanaxadiol and more effectively Protopanaxatriol are capable of binding to GR to activate its nuclear translocation, and to suppress the nuclear factor-kappa beta activity in GR-positive HeLa and HEK293 cells, but not in GR-low level COS-7 cells. Interestingly, no transactivation activity was observed, whereas suppression of the dexamethasone-induced transactivation of GR and induction of apoptosis in HeLa and HepG2 cells were observed. Thus, our results indicate that protopanaxadiol and Protopanaxatriol could be considered as potent and selective GR agonist.


