PMX 464Putative inhibitor of the Trx-TrxR system; antiproliferative CAS# 485842-97-5 |
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- BCX 1470
Catalog No.:BCC1413
CAS No.:217099-43-9
- BCX 1470 methanesulfonate
Catalog No.:BCC1414
CAS No.:217099-44-0
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 485842-97-5 | SDF | Download SDF |
| PubChem ID | 482697 | Appearance | Powder |
| Formula | C13H9NO2S | M.Wt | 243.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
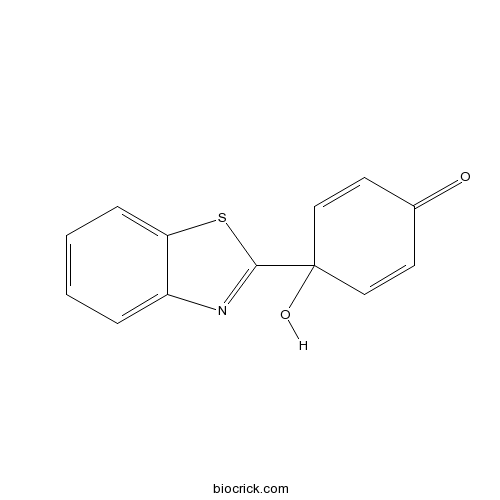
| Chemical Name | 4-(1,3-benzothiazol-2-yl)-4-hydroxycyclohexa-2,5-dien-1-one | ||
| SMILES | C1=CC=C2C(=C1)N=C(S2)C3(C=CC(=O)C=C3)O | ||
| Standard InChIKey | SDYBYKXWYDVVKP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H9NO2S/c15-9-5-7-13(16,8-6-9)12-14-10-3-1-2-4-11(10)17-12/h1-8,16H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Putative inhibitor of the thioredoxin-thioredoxin reductase (Trx-TrxR) system. Shown to inhibit Trx and induce a G1/S block in HT29 cells; inhibits cell proliferation in various colorectal cancer cell lines and MCF7 cells. Also elicits an anti-inflammatory response in A549 cells. |

PMX 464 Dilution Calculator

PMX 464 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1105 mL | 20.5524 mL | 41.1049 mL | 82.2098 mL | 102.7622 mL |
| 5 mM | 0.8221 mL | 4.1105 mL | 8.221 mL | 16.442 mL | 20.5524 mL |
| 10 mM | 0.411 mL | 2.0552 mL | 4.1105 mL | 8.221 mL | 10.2762 mL |
| 50 mM | 0.0822 mL | 0.411 mL | 0.8221 mL | 1.6442 mL | 2.0552 mL |
| 100 mM | 0.0411 mL | 0.2055 mL | 0.411 mL | 0.8221 mL | 1.0276 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mirabijalone D
Catalog No.:BCN4071
CAS No.:485811-84-5
- Choline sulphate
Catalog No.:BCN1792
CAS No.:4858-96-2
- Proanthocyanidins
Catalog No.:BCN6313
CAS No.:4852-22-6
- 5,7,3'-Trihydroxyflavanone
Catalog No.:BCC8269
CAS No.:104732-07-2
- Hydrangetin
Catalog No.:BCN7439
CAS No.:485-90-5
- Formononetin
Catalog No.:BCN1061
CAS No.:485-72-3
- Cinchonidine
Catalog No.:BCC5316
CAS No.:485-71-2
- (+)-Bicuculline
Catalog No.:BCN1238
CAS No.:485-49-4
- Cytisine
Catalog No.:BCN6270
CAS No.:485-35-8
- Reticuline
Catalog No.:BCN5583
CAS No.:485-19-8
- Aristolochic acid C
Catalog No.:BCN2658
CAS No.:4849-90-5
- N-Nornuciferine
Catalog No.:BCN4048
CAS No.:4846-19-9
- Isofraxidin
Catalog No.:BCN2327
CAS No.:486-21-5
- Fraxinol
Catalog No.:BCN5584
CAS No.:486-28-2
- Daphnetin
Catalog No.:BCN1051
CAS No.:486-35-1
- (S)-Coclaurine
Catalog No.:BCN5585
CAS No.:486-39-5
- (-)-Cotinine
Catalog No.:BCC7569
CAS No.:486-56-6
- Bergaptol
Catalog No.:BCN5588
CAS No.:486-60-2
- Ononin
Catalog No.:BCN5926
CAS No.:486-62-4
- Isoformononetin
Catalog No.:BCN8206
CAS No.:486-63-5
- Vasicinone
Catalog No.:BCN5589
CAS No.:486-64-6
- Daidzein
Catalog No.:BCN5590
CAS No.:486-66-8
- Harman
Catalog No.:BCN3998
CAS No.:486-84-0
- N-Methylcytisine
Catalog No.:BCN1266
CAS No.:486-86-2
Thioredoxin Inhibitors Attenuate Platelet Function and Thrombus Formation.[Pubmed:27716777]
PLoS One. 2016 Oct 7;11(10):e0163006.
Thioredoxin (Trx) is an oxidoreductase with important physiological function. Imbalances in the NADPH/thioredoxin reductase/thioredoxin system are associated with a number of pathologies, particularly cancer, and a number of clinical trials for thioredoxin and thioredoxin reductase inhibitors have been carried out or are underway. Due to the emerging role and importance of oxidoreductases for haemostasis and the current interest in developing inhibitors for clinical use, we thought it pertinent to assess whether inhibition of the NADPH/thioredoxin reductase/thioredoxin system affects platelet function and thrombosis. We used small molecule inhibitors of Trx (PMX 464 and PX-12) to determine whether Trx activity influences platelet function, as well as an unbiased proteomics approach to identify potential Trx substrates on the surface of platelets that might contribute to platelet reactivity and function. Using LC-MS/MS we found that PMX 464 and PX-12 affected the oxidation state of thiols in a number of cell surface proteins. Key surface receptors for platelet adhesion and activation were affected, including the collagen receptor GPVI and the von Willebrand factor receptor, GPIb. To experimentally validate these findings we assessed platelet function in the presence of PMX 464, PX-12, and rutin (a selective inhibitor of the related protein disulphide isomerase). In agreement with the proteomics data, small molecule inhibitors of thioredoxin selectively inhibited GPVI-mediated platelet activation, and attenuated ristocetin-induced GPIb-vWF-mediated platelet agglutination, thus validating the findings of the proteomics study. These data reveal a novel role for thioredoxin in regulating platelet reactivity via proteins required for early platelet responses at sites of vessel injury (GPVI and GPIb). This work also highlights a potential opportunity for repurposing of PMX 464 and PX-12 as antiplatelet agents.
Basal and angiotensin II-inhibited neuronal delayed-rectifier K+ current are regulated by thioredoxin.[Pubmed:17360810]
Am J Physiol Cell Physiol. 2007 Jul;293(1):C211-7.
In previous studies, we determined that macrophage migration inhibitory factor (MIF), acting intracellularly via its intrinsic thiol-protein oxidoreductase (TPOR) activity, stimulates basal neuronal delayed-rectifier K(+) current (I(Kv)) and inhibits basal and angiotensin (ANG) II-induced increases in neuronal activity. These findings are the basis for our hypothesis that MIF is a negative regulator of ANG II actions in neurons. MIF has recently been recategorized as a member of the thioredoxin (Trx) superfamily of small proteins. In the present study we have examined whether Trx influences basal and ANG II-modulated I(Kv) in an effort to determine whether the Trx superfamily can exert a general regulatory influence over neuronal activity and the actions of ANG II. Intracellular application of Trx (0.8-80 nM) into rat hypothalamic/brain stem neurons in culture increased neuronal I(Kv), as measured by voltage-clamp recordings. This effect of Trx was abolished in the presence of the TPOR inhibitor PMX 464 (800 nM). Furthermore, the mutant protein recombinant human C32S/C35S-Trx, which lacks TPOR activity, failed to alter neuronal I(Kv). Trx applied at a concentration (0.08 nM) that does not alter basal I(Kv) abolished the inhibition of neuronal I(Kv) produced by ANG II (100 nM). Given our observation that ANG II increases Trx levels in neuronal cultures, it is possible that Trx (like MIF) has a negative regulatory role over basal and ANG II-stimulated neuronal activity via modulation of I(Kv). Moreover, these data suggest that TPOR may be a general mechanism for negatively regulating neuronal activity.
Antitumour properties of fluorinated benzothiazole-substituted hydroxycyclohexa-2,5-dienones ('quinols').[Pubmed:16908135]
Bioorg Med Chem Lett. 2006 Oct 1;16(19):5005-8.
The synthesis and in vitro antitumour evaluation of a new series of fluorinated benzothiazole-substituted 4-hydroxycyclohexa-2,5-dienones ('quinols') is described. The new compounds were found to be of comparable activity compared to the non-fluorinated precursor PMX 464, in terms of antiproliferative activity in sensitive human cancer cell lines (nanomolar GI(50) values) and inhibitory activity against the thioredoxin signalling system.
PMX464, a thiol-reactive quinol and putative thioredoxin inhibitor, inhibits NF-kappaB-dependent proinflammatory activation of alveolar epithelial cells.[Pubmed:18587424]
Br J Pharmacol. 2008 Nov;155(5):661-72.
BACKGROUND AND PURPOSE: Subtle changes in the intracellular reduction-oxidation (redox) state can modulate nuclear factor-kappaB (NF-kappaB) activity. Thioredoxin-1 (Trx) is a small, ubiquitous, redox-active thiol (-SH) protein that, with thioredoxin reductase-1 (TrxR), modifies the redox status of NF-kappaB pathway components. PMX464 is a novel thiol-reactive quinol thought to inhibit the Trx/TrxR system. The aim of this work was to investigate whether PMX464 inhibited NF-kappaB-mediated proinflammatory activation of human type II alveolar epithelial cells (A549). EXPERIMENTAL APPROACH: Intercellular adhesion molecule-1 (ICAM-1), granulocyte-macrophage colony-stimulating factor (GM-CSF) and CXCL8, NF-kappaB DNA binding, nuclear translocation of NF-kappaB p65 subunit, IkappaBalpha degradation, IkappaB phosphorylation and IkappaB kinase (IKK) activity were assessed in A549 cells stimulated with IL-1beta with or without PMX464 pretreatment. Effects of PMX464 on ICAM-1 expression in human lung microvascular endothelial cells (HLMVEC) were also investigated. For comparison, selected measurements (ICAM-1 and IkappaB-alpha phospho-IkappaB-alpha) were made on A549 cells after RNA interference-mediated silencing (siRNA) of Trx. KEY RESULTS: PMX464 reduced ICAM-1, GM-CSF and CXCL8 expression in IL-1beta-stimulated A549 cells and ICAM-1 in HLMVEC. PMX464 inhibited IL-1beta-induced NF-kappaB DNA binding, nuclear translocation of NF-kappaB p65 subunit and factors involved in NF-kappaB activation; specifically, IkappaBalpha degradation, IkappaB phosphorylation and IkappaB kinase (IKK) activity in A549. By contrast, Trx siRNA did not alter ICAM-1 expression or IkappaBalpha degradation/phosphorylation in IL-1beta-stimulated A549 cells. CONCLUSION AND IMPLICATIONS: PMX464 inhibits a proinflammatory response in A549 cells targeting the NFkappaB pathway above IKK. The lack of effect with Trx siRNA suggests that PMX464 acts on thiol proteins, in addition to Trx, to elicit anti-inflammatory responses in lung epithelial cells.
A cellular and molecular investigation of the action of PMX464, a putative thioredoxin inhibitor, in normal and colorectal cancer cell lines.[Pubmed:17572693]
Br J Pharmacol. 2007 Aug;151(8):1167-75.
BACKGROUND AND PURPOSE: PMX464 is a novel benzothiazole substituted cyclohexadienone reportedly targeting the thioredoxin (Trx1)/thioredoxin reductase (TrxR1) system. We have previously shown that PMX464 has enhanced hypoxic anti-proliferative effects in colorectal tumour cells, with some non-tumour cells (quiescent endothelium and fibroblasts) being relatively resistant. The current study aimed to validate the Trx1 system as a molecular target of PMX464 in tumour cells and to investigate the differential sensitivities of normal cells at the molecular level. EXPERIMENTAL APPROACH: Proliferation, clonogenic survival, protein expression and function, cell cycle and apoptosis assays were conducted using colorectal tumour (HT29), endothelial (HUVEC) and fibroblast (MRCV) cells treated with PMX464 under normoxic and hypoxic (1% O(2)) conditions. KEY RESULTS: Protein and enzyme assays showed that PMX464, in HT29, inhibited Trx1 function without altering expression and that inhibition correlated with decreased proliferation and survival, and was more marked under hypoxia. In contrast, although hypoxic HUVEC were sensitive, in terms of proliferation and survival, inhibition of Trx1 function was not observed. Quiescent HUVEC and MRCVs (that have undetectable Trx1 protein) were relatively resistant. The effect on HT29 cells was essentially due to cell cycle inhibition, as apoptosis was modest. Anti-proliferative effects were lost after a lag period, suggesting a reversible phenomenon. CONCLUSIONS AND IMPLICATIONS: The Trx1 system is an important target in tumour cells and can be inhibited by PMX464. Quiescent HUVEC and fibroblasts are relatively resistant conferring a therapeutic benefit when targeting Trx1.
Cytotoxic and antiangiogenic activity of AW464 (NSC 706704), a novel thioredoxin inhibitor: an in vitro study.[Pubmed:15655539]
Br J Cancer. 2005 Jan 31;92(2):350-8.
AW464 (NSC 706704) is a novel benzothiazole substituted quinol compound active against colon, renal and certain breast cancer cell lines. NCI COMPARE analysis indicates possible interaction with thioredoxin/thioredoxin reductase, which is upregulated under hypoxia. Through activity on HIF1alpha, VEGF levels are regulated and angiogenesis controlled. A thioredoxin inhibitor could therefore exhibit enhanced hypoxic toxicity and indirect antiangiogenic effects. In vitro experiments were performed on colorectal and breast cancer cell lines under both normoxic and hypoxic conditions and results compared against those obtained with normal cell lines, fibroblasts and keratinocytes. Antiangiogenic effects were studied using both large and microvessel cells. Indirect antiangiogenic effects (production of angiogenic growth factors) were studied via ELISA. We show that AW464 exerts antiproliferative effects on tumour cell lines as well as endothelial cells with an IC(50) of approximately 0.5 microM. Fibroblasts are however resistant. Proliferating, rather than quiescent, endothelial cells are sensitive to the drug indicating potential antiangiogenic rather than antivascular action. Endothelial differentiation is also inhibited in vitro. Hypoxia (1% O(2) for 48 h) sensitises colorectal cells to lower drug concentrations, and in HT29s greater inhibition of VEGF is observed under such conditions. In contrast, bFGF levels are unaffected, suggesting possible involvement of HIF1alpha. Thus, AW464 is a promising chemotherapeutic drug that may have enhanced potency under hypoxic conditions and also additional antiangiogenic activity.


