(S)-CoclaurineCAS# 486-39-5 |
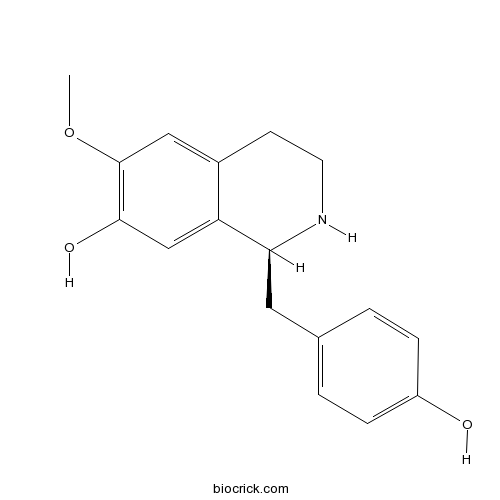
- (R)-Coclaurine
Catalog No.:BCN8348
CAS No.:2196-60-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 486-39-5 | SDF | Download SDF |
| PubChem ID | 160487 | Appearance | Powder |
| Formula | C17H19NO3 | M.Wt | 285.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S)-1-[(4-hydroxyphenyl)methyl]-6-methoxy-1,2,3,4-tetrahydroisoquinolin-7-ol | ||
| SMILES | COC1=C(C=C2C(NCCC2=C1)CC3=CC=C(C=C3)O)O | ||
| Standard InChIKey | LVVKXRQZSRUVPY-HNNXBMFYSA-N | ||
| Standard InChI | InChI=1S/C17H19NO3/c1-21-17-9-12-6-7-18-15(14(12)10-16(17)20)8-11-2-4-13(19)5-3-11/h2-5,9-10,15,18-20H,6-8H2,1H3/t15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (+)-R-Coclaurine and (+)-S-reticuline show negative inotropic effects. 2. Coclaurine derivatives and of paeoniflorin derivatives have neuromuscular blocking actions. 3. D-Coclaurine has a neuroleptic-like property in blocking effects of dopaminergic stimulating agents. |
| Targets | Calcium Channel |

(S)-Coclaurine Dilution Calculator

(S)-Coclaurine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5051 mL | 17.5254 mL | 35.0508 mL | 70.1016 mL | 87.6271 mL |
| 5 mM | 0.701 mL | 3.5051 mL | 7.0102 mL | 14.0203 mL | 17.5254 mL |
| 10 mM | 0.3505 mL | 1.7525 mL | 3.5051 mL | 7.0102 mL | 8.7627 mL |
| 50 mM | 0.0701 mL | 0.3505 mL | 0.701 mL | 1.402 mL | 1.7525 mL |
| 100 mM | 0.0351 mL | 0.1753 mL | 0.3505 mL | 0.701 mL | 0.8763 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Daphnetin
Catalog No.:BCN1051
CAS No.:486-35-1
- Fraxinol
Catalog No.:BCN5584
CAS No.:486-28-2
- Isofraxidin
Catalog No.:BCN2327
CAS No.:486-21-5
- PMX 464
Catalog No.:BCC6348
CAS No.:485842-97-5
- Mirabijalone D
Catalog No.:BCN4071
CAS No.:485811-84-5
- Choline sulphate
Catalog No.:BCN1792
CAS No.:4858-96-2
- Proanthocyanidins
Catalog No.:BCN6313
CAS No.:4852-22-6
- 5,7,3'-Trihydroxyflavanone
Catalog No.:BCC8269
CAS No.:104732-07-2
- Hydrangetin
Catalog No.:BCN7439
CAS No.:485-90-5
- Formononetin
Catalog No.:BCN1061
CAS No.:485-72-3
- Cinchonidine
Catalog No.:BCC5316
CAS No.:485-71-2
- (+)-Bicuculline
Catalog No.:BCN1238
CAS No.:485-49-4
- (-)-Cotinine
Catalog No.:BCC7569
CAS No.:486-56-6
- Bergaptol
Catalog No.:BCN5588
CAS No.:486-60-2
- Ononin
Catalog No.:BCN5926
CAS No.:486-62-4
- Isoformononetin
Catalog No.:BCN8206
CAS No.:486-63-5
- Vasicinone
Catalog No.:BCN5589
CAS No.:486-64-6
- Daidzein
Catalog No.:BCN5590
CAS No.:486-66-8
- Harman
Catalog No.:BCN3998
CAS No.:486-84-0
- N-Methylcytisine
Catalog No.:BCN1266
CAS No.:486-86-2
- alpha-Isolupanine
Catalog No.:BCN7989
CAS No.:486-87-3
- Anagyrine
Catalog No.:BCN3049
CAS No.:486-89-5
- Thermopsine
Catalog No.:BCN2603
CAS No.:486-90-8
- AZD2858
Catalog No.:BCC4509
CAS No.:486424-20-8
Effects of d-coclaurine and d-reticuline, benzyltetrahydroisoquinoline alkaloids, on levels of 3,4-dihydroxyphenylacetic acid and homovanillic acid in the mouse striatum.[Pubmed:6141236]
J Pharmacobiodyn. 1983 Oct;6(10):793-6.
An intracerebroventricular injection of d-coclaurine (50 micrograms), a benzyltetrahydroisoquinoline alkaloid extracted from Magnolia salicifolia, produced a slight increase in 3,4-dihydroxyphenylacetic acid level and a significant increase in homovanillic acid level in the mouse striatum. Another alkaloid d-reticuline (200 micrograms) increased only homovanillic acid level. An intracerebroventricular pretreatment with d-coclaurine (50 micrograms) did not antagonize suppressive effect of apomorphine on l-dopa formation produced by gamma-butyrolactone (750 mg/kg i.p.) plus aromatic amino acid decarboxylase inhibitor, NSD-1015 (100 mg/kg i.p.). These results suggest that d-coclaurine blocks postsynaptic but not presynaptic dopamine receptors in the mouse striatum.
Inotropic effects of (+/-)-higenamine and its chemically related components, (+)-R-coclaurine and (+)-S-reticuline, contained in the traditional sino-Japanese medicines "bushi" and "shin-i" in isolated guinea pig papillary muscle.[Pubmed:2724702]
Jpn J Pharmacol. 1989 May;50(1):75-8.
(+/-)-Higenamine (Hig. demethylcoclaurine) is a cardiotonic principle from aconite root. (+)-R-Coclaurine (Coc) and (+)-S-reticuline (Ret) are compounds contained in the dried buds of Magnolia salicifolia MAXIM. All of these alkaloids possess a common chemical structure: tetrahydroisoquinoline. Coc and Ret showed negative inotropic effects in contrast to the positive inotropic effects of Hig in papillary muscles of guinea pigs. Coc and Ret shifted to the right the concentration-contraction curves of Hig. Hig shifted in parallel to the left the Ca2+ curve, and it tended to shift to the left the isoproterenol (Isp)-induced response curve. In contrast, Coc and Ret inhibited the Ca2+ curve and the low concentration range of the Isp-induced curve, and it potentiated the high concentration ranges of Ca2+ and Isp. Coc and Ret showed actions that were reversed in direction to those of Hig, as clearly demonstrated in the Ca2+ curve.
Purification and characterization of coclaurine N-methyltransferase from cultured Coptis japonica cells.[Pubmed:11314949]
Phytochemistry. 2001 Apr;56(7):649-55.
S-Adenosyl-L-methionine (SAM): coclaurine N-methyltransferase (CNMT), which catalyzes the transfer of a methyl group from S-adenosyl-L-methionine to the amino group of the tetrahydrobenzylisoquinoline alkaloid coclaurine. was purified 340-fold from Coptis japonica cells in 1% yield to give an almost homogeneous protein. The purified enzyme, which occurred as a homotetramer with a native Mr of 160 kDa (gel-filtration chromatography) and a subunit Mr of 45 kDa (SDS-polyacrylamide gel electrophoresis), had an optimum pH of 7.0 and a pI of 4.2. Whereas (R)-coclaurine was the best substrate for enzyme activity, Coptis CNMT had broad substrate specificity and no stereospecificity CNMT methylated norlaudanosoline, 6,7-dimethoxyl-1,2,3,4-tetrahydroisoquinoline and 1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline. The enzyme did not require any metal ion. p-Chloromercuribenzoate and iodoacetamide did not inhibit CNMT activity, but the addition of Co2+, Cu2+ or Mn2+ at 5 mM severely inhibited such activity by 75, 47 and 57%, respectively. The substrate-saturation kinetics of CNMT for norreticuline and SAM were of the typical Michaelis-Menten-type with respective Km values of 0.38 and 0.65 mM.


