Ondansetron HCl5-HT3 receptor antagonist CAS# 99614-01-4 |
- GNF-5837
Catalog No.:BCC3668
CAS No.:1033769-28-6
- Lestaurtinib
Catalog No.:BCC2440
CAS No.:111358-88-4
- Sanguinarine
Catalog No.:BCN5102
CAS No.:2447-54-3
- Astilbin
Catalog No.:BCN5204
CAS No.:29838-67-3
- Arctigenin
Catalog No.:BCN6291
CAS No.:7770-78-7
- Teriflunomide
Catalog No.:BCN9645
CAS No.:163451-81-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
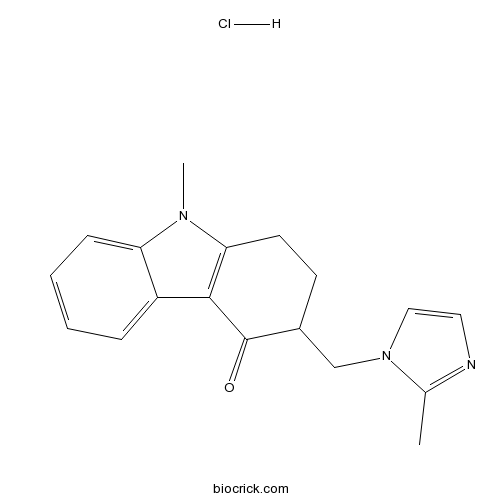
| Cas No. | 99614-01-4 | SDF | Download SDF |
| PubChem ID | 68647 | Appearance | Powder |
| Formula | C18H20ClN3O | M.Wt | 329.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GR 38032 | ||
| Solubility | Soluble to 50 mM in water and to 100 mM in DMSO | ||
| Chemical Name | 1,2,3,9-Tetrahydro-9-methyl-3-[(2-m | ||
| SMILES | CC1=NC=CN1CC2CCC3=C(C2=O)C4=CC=CC=C4N3C.Cl | ||
| Standard InChIKey | MKBLHFILKIKSQM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H19N3O.ClH/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2;/h3-6,9-10,13H,7-8,11H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective 5-HT3 receptor antagonist (Ki = 6.16 nM). Antiemetic; prevents emesis induced by cytotoxic drugs and radiation. |

Ondansetron HCl Dilution Calculator

Ondansetron HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.032 mL | 15.1598 mL | 30.3196 mL | 60.6391 mL | 75.7989 mL |
| 5 mM | 0.6064 mL | 3.032 mL | 6.0639 mL | 12.1278 mL | 15.1598 mL |
| 10 mM | 0.3032 mL | 1.516 mL | 3.032 mL | 6.0639 mL | 7.5799 mL |
| 50 mM | 0.0606 mL | 0.3032 mL | 0.6064 mL | 1.2128 mL | 1.516 mL |
| 100 mM | 0.0303 mL | 0.1516 mL | 0.3032 mL | 0.6064 mL | 0.758 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ondansetron hydrochloride dihydrate is a serotonin-3 (5-HT3) receptor antagonist.
- Sertaconazole nitrate
Catalog No.:BCC4716
CAS No.:99592-39-9
- Sertaconazole
Catalog No.:BCC9146
CAS No.:99592-32-2
- Neuropeptide FF
Catalog No.:BCC5983
CAS No.:99566-27-5
- K 252a
Catalog No.:BCC7152
CAS No.:99533-80-9
- L-651,582
Catalog No.:BCC7561
CAS No.:99519-84-3
- 3-Ethoxy-4-ethoxycarbonyl phenylacetic acid
Catalog No.:BCC8629
CAS No.:99469-99-5
- 1-Chloroethyl cyclohexyl carbonate
Catalog No.:BCC8463
CAS No.:99464-83-2
- Ampiroxicam
Catalog No.:BCC4426
CAS No.:99464-64-9
- BTZO 1
Catalog No.:BCC7886
CAS No.:99420-15-2
- Methyl rosmarinate
Catalog No.:BCN4536
CAS No.:99353-00-1
- Kadsurin A
Catalog No.:BCN6515
CAS No.:99340-07-5
- Venlafaxine Hydrochloride
Catalog No.:BCC2513
CAS No.:99300-78-4
- Ondansetron
Catalog No.:BCC5043
CAS No.:99614-02-5
- Leucanthogenin
Catalog No.:BCN7932
CAS No.:99615-00-6
- Isothymonin
Catalog No.:BCN3393
CAS No.:99615-01-7
- Kazinol B
Catalog No.:BCN4538
CAS No.:99624-27-8
- Kazinol A
Catalog No.:BCN3388
CAS No.:99624-28-9
- ent-3beta,18-Dihydroxylabda-8(17),13E-dien-15-oic acid
Catalog No.:BCN7669
CAS No.:99624-39-2
- Uncinatone
Catalog No.:BCN4547
CAS No.:99624-92-7
- Ro 19-4603
Catalog No.:BCC7228
CAS No.:99632-94-7
- 14-Benzoylneoline
Catalog No.:BCN6493
CAS No.:99633-05-3
- Scholaricine
Catalog No.:BCN4539
CAS No.:99694-90-3
- Rotigotine
Catalog No.:BCC1907
CAS No.:99755-59-6
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
Surface engineered nanostructured lipid carriers for efficient nose to brain delivery of ondansetron HCl using Delonix regia gum as a natural mucoadhesive polymer.[Pubmed:25033434]
Colloids Surf B Biointerfaces. 2014 Oct 1;122:143-150.
The objective of this investigation was to fabricate ondansetron hydrochloride [OND] loaded mucoadhesive nanostructured lipid carriers [NLCs] for efficient delivery to brain through nasal route. Mucoadhesive NLCs thereby sustaining drug release for longer time in nasal cavity. NLCs were prepared by high pressure homogenization [HPH] technique using glycerol monostearate [GMS]; as solid lipid, Capryol 90; as liquid lipid, soya lecithin; as surfactant and poloxamer 188; as cosurfactant. In the fabrication of NLCs, Delonix regia gum [DRG], isolated from seeds of D. regia belonging to family fabiaceae was used as a mucoadhesive polymer. The NLCs were evaluated for particle size, morphology, drug-entrapment efficiency [%EE], mucoadhesive strength, in vitro drug release, histological examination, ex vivo permeation study, in vivo biodistribution and pharmacokinetic studies in the brain/blood following intravenous [i.v.] and intranasal [i.n.] administration. Particle size, PDI, Zeta potential was observed in the range of 92.28-135nm, 0.32-0.46, and -11.5 to -36.2 respectively. Prepared NLCs achieved thermodynamic stability, control release pattern with minor histopathological changes in sheep nasal mucosa. The significantly [P<0.05] higher values for selected batch was observed, when administered by i.n. route showed higher drug targeting efficiency [506%] and direct transport percentage [97.14%] which confirms the development of promising OND-loaded NLC for efficient nose-to-brain delivery.
Solid lipid nanoparticles of ondansetron HCl for intranasal delivery: development, optimization and evaluation.[Pubmed:22802103]
J Mater Sci Mater Med. 2012 Sep;23(9):2163-75.
The present investigation deals with the development and statistical optimization of solid lipid nanoparticles (SLNs) of Ondansetron HCl (OND) for intranasal (i.n.) delivery. SLNs were prepared using the solvent diffusion technique and a 2(3) factorial design. The concentrations of lipid, surfactant and cosurfactant were independent variables in this design, whereas, particle size and entrapment efficiency (EE) were dependent variables. The particle size of the SLNs was found to be 320-498 nm, and the EE was between 32.89 and 56.56 %. The influence of the lipid, surfactant and cosurfactant on the particle size and EE was studied. A histological study revealed no adverse response of SLNs on sheep nasal mucosa. Transmission electron microscopic analysis showed spherical shape particles. Differential scanning calorimetry and X-ray diffraction studies indicated that the drug was completely encapsulated in a lipid matrix. In vitro drug release studies carried out in phosphate buffer (pH 6.6) indicated that the drug transport was of Fickian type. Gamma scintigraphic imaging in rabbits after i.n. administration showed rapid localization of the drug in the brain. Hence, OND SLNs is a promising nasal delivery system for rapid and direct nose-to-brain delivery.
Formulation and evaluation of lipid based taste masked granules of ondansetron HCl.[Pubmed:24905829]
Eur J Pharm Sci. 2014 Oct 1;62:180-8.
INTRODUCTION AND AIM: Various taste masking approaches comprising the excipients which delay the reach of the drug to taste buds are reported. Lipidic substances can act as release retarding agent and provides a matrix base responsible for suppressing the bitter taste of drug. This work was aimed to study the influence of different proportions of a lipid carrier on the inhibition of bitterness of the drug vis-a-vis in vitro release of drug from the granules. METHODS: The lipid-matrix granules of Ondansetron HCl with Geleol pellets (glycerol monostearate) were obtained by manual hot melt fusion technique. The prepared granules were characterized by SEM, DSC and XRD. The taste assessment of prepared granules was done by in vitro method based on drug release. RESULTS: Distribution of drug inside the lipid-matrix granules was not properly analyzed by DSC and XRD, moreover these studies revealed no interaction between the drug and lipid. The dissolution tests displayed the significant retardation of drug release from the granules compared to pure drug and additionally indicated the attainment of matrix system via appearance of unbroken granules during in vitro testing. Higuchi relationship for drug release was obtained by drug release kinetics, which also revealed the functioning drug release mechanism, as diffusion controlled but the addition of hydrophilic substance (Cab-o-sil) has changed the mechanism of drug release. CONCLUSION: The proportions of Geleol and Cab-o-sil taken in granules had affected the dissolution profile. Higher amount of GE resulted in high taste masking ability.
Formulation and development of pH-independent/dependent sustained release matrix tablets of ondansetron HCl by a continuous twin-screw melt granulation process.[Pubmed:25863118]
Int J Pharm. 2015 Dec 30;496(1):33-41.
The objective of the present study was to develop pH-independent/dependent sustained release (SR) tablets of Ondansetron HCl dihydrate (OND), a selective 5-HT3 receptor antagonist that is used for prevention of nausea and vomiting caused by chemotherapy, radiotherapy and postoperative treatment. The challenge with the OND API is its pH-dependent solubility and relatively short elimination half-life. Therefore, investigations were made to solve these problems in the current study. Formulations were prepared using stearic acid as a binding agent via a melt granulation process in a twin-screw extruder. The micro-environmental pH of the tablet was manipulated by the addition of fumaric acid to enhance the solubility and release of OND from the tablet. The in vitro release study demonstrated sustained release for 24h with 90% of drug release in formulations using stearic acid in combination with ethyl cellulose, whereas 100% drug release in 8h for stearic acid-hydroxypropylcellulose matrices. The formulation release kinetics was correlated to the Higuchi diffusion model and a non-Fickian drug release mechanism. The results of the present study demonstrated for the first time the pH dependent release from hydrophilic-lipid matrices as well as pH independent release from hydrophobic-lipid matrices for OND SR tablets manufactured by means of a continuous melt granulation technique utilizing a twin-screw extruder.
Ondansetron, a selective 5-HT3 antagonist, antagonizes methamphetamine-induced anorexia in mice.[Pubmed:15661576]
Pharmacol Res. 2005 Mar;51(3):255-9.
Effects of some selective serotonergic (5-HT) antagonists on methamphetamine-induced anorexia were investigated in male mice. The least possible dose of methamphetamine alone that caused significant anorectic activity was 11 micromolkg(-1), i.p. (2 mgkg(-1)). Various doses of some selective serotonergic receptor antagonists were administered half an hour before the above mentioned dose of methamphetamine. Methiothepin potentiated, whereas NAN-190, methysergide, mianserin and ondansetron antagonized methamphetamine-induced anorectic activity. The least possible doses of these antagonists which modified methamphetamine-induced anorexia were as follows: methiothepin (1.1 micromolkg(-1), i.p.), NAN-190 (4.2 micromolkg(-1), i.p.), methysergide (2.1 micromolkg(-1), i.p.), mianserin (3.3 micromolkg(-1), i.p.) and ondansetron (0.003 micromolkg(-1), i.p.). The serotonergic antagonists at the above mentioned doses did not modify the food intake of animals not treated with methamphetamine, except for methiothepin, which produced a significant reduction, and mianserin, which produced a significant increase in food intake. The results of the present study indicated that the anorectic activity induced by methamphetamine is related to the interactions of methamphetamine with 5-HT receptor. Since a very small dose (0.003 micromolkg(-1)) of ondansetron (the 5-HT(3) antagonist), as compared with the other antagonists used in this study, antagonized the anorexia induced by methamphetamine, the 5-HT(3) receptor is likely to be the site for this interaction.
Ondansetron: a selective 5-HT(3) receptor antagonist and its applications in CNS-related disorders.[Pubmed:11474424]
CNS Drug Rev. 2001 Summer;7(2):199-213.
Ondansetron is a selective 5-hydroxytryptamine(3) (5-HT(3)) receptor antagonist that has been introduced to clinical practice as an antiemetic for cancer treatment-induced and anesthesia-related nausea and vomiting. Its use under these circumstances is both prophylactic and therapeutic. It has a superior efficacy, safety and pharmacoeconomic profile compared with other groups of antiemetics, namely antidopaminergics, antihistamines and anticholinergics. However, its place in the management of anticipatory and delayed vomiting in cancer treatment and as a rescue antiemetic in surgical patients needs to be further explored. Furthermore, recent animal and human research also reflects its possible novel application in the treatment of other disease states, such as alcoholism, cocaine addiction, opioid withdrawal syndrome, anxiety disorders, gastrointestinal motility disorders, Tourette's syndrome and pruritus. This review revisits the widespread physiological and pathological effects of 5-HT and discusses both the basic science literature and the clinical developments responsible for the conventional and novel uses of ondansetron. In addition, new discoveries relating to the effects of ondansetron on other receptors/channels and their possible therapeutic applications are presented.
Development of high-affinity 5-HT3 receptor antagonists. 1. Initial structure-activity relationship of novel benzamides.[Pubmed:1312602]
J Med Chem. 1992 Mar 6;35(5):895-903.
This report describes the development of novel benzamides which are orally active, highly potent, specific antagonists of 5-HT3 receptors. Described in this first report are the structure-activity relationships that led to novel structures with improved potency and selectivity. From this series of compounds, (S)-28 was identified and selected for further evaluation as a 5-HT3 receptor antagonist. Compared with 5-HT3 antagonists such as GR 38032F, BRL 43694, and metoclopramide, (S)-28 was most active in (a) inhibiting binding to 5-HT3 receptor binding sites in rat entorhinal cortex with an Ki value of 0.19 nM and (b) blocking cisplatin-induced emesis in the ferret with an ED50 value determined to be 9 micrograms/kg po.


