Oligomycin AMitochondrial ATP synthase Inhibitor CAS# 579-13-5 |
- ARL 67156 trisodium salt
Catalog No.:BCC7004
CAS No.:1021868-83-6
- Brefeldin A
Catalog No.:BCC4387
CAS No.:20350-15-6
- BTB06584
Catalog No.:BCC5106
CAS No.:219793-45-0
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
Quality Control & MSDS
Number of papers citing our products

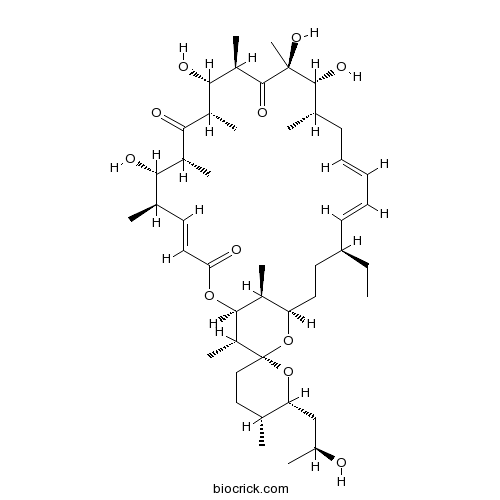
Chemical structure
3D structure
| Cas No. | 579-13-5 | SDF | Download SDF |
| PubChem ID | 5281899 | Appearance | Powder |
| Formula | C45H74O11 | M.Wt | 791.06 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (158.02 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (1R,4S,5E,5'R,6'R,7E,10S,11R,12S,14R,15S,16S,18R,19S,20R,21E,25S,26R,27S,29S)-4-ethyl-11,12,15,19-tetrahydroxy-6'-[(2S)-2-hydroxypropyl]-5',10,12,14,16,18,20,26,29-nonamethylspiro[24,28-dioxabicyclo[23.3.1]nonacosa-5,7,21-triene-27,2'-oxane]-13,17,23-trione | ||
| SMILES | CCC1CCC2C(C(C(C3(O2)CCC(C(O3)CC(C)O)C)C)OC(=O)C=CC(C(C(C(=O)C(C(C(C(=O)C(C(C(CC=CC=C1)C)O)(C)O)C)O)C)C)O)C)C | ||
| Standard InChIKey | MNULEGDCPYONBU-WMBHJXFZSA-N | ||
| Standard InChI | InChI=1S/C45H74O11/c1-12-34-17-15-13-14-16-27(4)42(51)44(11,53)43(52)32(9)40(50)31(8)39(49)30(7)38(48)26(3)18-21-37(47)54-41-29(6)35(20-19-34)55-45(33(41)10)23-22-25(2)36(56-45)24-28(5)46/h13-15,17-18,21,25-36,38,40-42,46,48,50-51,53H,12,16,19-20,22-24H2,1-11H3/b14-13+,17-15+,21-18+/t25-,26-,27+,28+,29+,30-,31-,32-,33-,34-,35-,36-,38+,40+,41+,42-,44+,45-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of mitochondrial ATP synthase and uncoupler of oxidative phosphorylation. Antibiotic; exhibits anti-tumor activity. |

Oligomycin A Dilution Calculator

Oligomycin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2641 mL | 6.3206 mL | 12.6413 mL | 25.2825 mL | 31.6032 mL |
| 5 mM | 0.2528 mL | 1.2641 mL | 2.5283 mL | 5.0565 mL | 6.3206 mL |
| 10 mM | 0.1264 mL | 0.6321 mL | 1.2641 mL | 2.5283 mL | 3.1603 mL |
| 50 mM | 0.0253 mL | 0.1264 mL | 0.2528 mL | 0.5057 mL | 0.6321 mL |
| 100 mM | 0.0126 mL | 0.0632 mL | 0.1264 mL | 0.2528 mL | 0.316 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Oligomycin A is an inhibitor of ATP synthase, which inhibits the process taking place on mitochondria coupling membrane that depended on ATP and oxidative phosphorylation [1].
Oligomycin A blocks proton channel of ATP synthase, which is necessary for transforming ADP to ATP by oxidative phosphorylation, accomplishing the inhibition of ATP synthase. The process which oligomycin A inhibits ATP synthesis can significantly reduce electron flow through the electron transport chain.
Experiments which performed in testing against 60 human cancer cell lines that from the National Cancer Institute showed that oligomycin is among the top 0.1% of the most cell line selective cytotoxic agents of 37,000 molecules. In the HeLa carcinoma cell line, the inhibitors of H+ -ATP-synthase oligomycin (5 mg/ml) was shown to strongly suppress, and the cell respiration, showing that it is tightly coupled to ATP synthesis. It was reported that Oligomycin at 100 ng/ml completely inhibits the activity of oxidative phosphorylation in 1h and induces different levels of glycolysis gains by 6 h in a group of cancer cell. As an inhibitor of the F0 part of H+-ATP-synthase, Oligomycin also suppresses the apoptosis which was induced by TNF. Treatment with different concentrations of oligomycin and rotenone severely reduced the oxygen consumption by up to 94%, indicating a major role for mitochondria in this process. And treatment with oligomycin could abolish the H2O2 increase completely [2-4].
References:
[1]. Jastroch M, Divakaruni AS, Mookerjee S, et al. Mitochondrial proton and electron leaks. Essays Biochemistry, 2010, 47:53-67.
[2]. Shchepina LA, Pletjushkina OY, Avetisyan AV, et al. Oligomycin, inhibitor of the F-0 part of H+-ATP-synthase, suppresses the TNF-induced apoptosis. Oncogene, 2002, 53: 8149-8157.
[3]. Salomon AR, Voehringer DW, Herzenberg LA, et al. Understanding and exploiting the mechanistic basis for selectivity of polyketide inhibitors of F0F1-ATPase. Proceedings of The National Academy of Sciences of The United States of America, 2000, 97(26): 14766-14771.
[4]. Alexander R, Adina V, Ivan B, et al. Overcoming intrinsic multi-drug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1Bhigh cells. Cancer Cell, 2013, 23(6): 811-825.
- Myrianthic acid 3,23-acetonide
Catalog No.:BCN7517
CAS No.:578710-52-8
- Clozapine
Catalog No.:BCC5037
CAS No.:5786-21-0
- Idarubicin HCl
Catalog No.:BCC1194
CAS No.:57852-57-0
- Stevioside
Catalog No.:BCN6305
CAS No.:57817-89-7
- Domperidone
Catalog No.:BCC4461
CAS No.:57808-66-9
- Liquiritigenin
Catalog No.:BCN5946
CAS No.:578-86-9
- Cosmosiin
Catalog No.:BCN5788
CAS No.:578-74-5
- 8-Aminoquinoline
Catalog No.:BCC8784
CAS No.:578-66-5
- 4-(2-Hydroxy-1-methoxyethyl)-1,2-benzenediol
Catalog No.:BCN1412
CAS No.:577976-26-2
- Topiroxostat
Catalog No.:BCC4202
CAS No.:577778-58-6
- Cardionogen 1
Catalog No.:BCC6199
CAS No.:577696-37-8
- WAY 629 hydrochloride
Catalog No.:BCC7271
CAS No.:57756-44-2
- Lobelanine
Catalog No.:BCN2156
CAS No.:579-21-5
- o-Anisic acid
Catalog No.:BCC9108
CAS No.:579-75-9
- 19-Nor-4-hydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1411
CAS No.:57906-31-7
- Corynoxidine
Catalog No.:BCN6798
CAS No.:57906-85-1
- Z-Cys(Z)-OH
Catalog No.:BCC2784
CAS No.:57912-35-3
- L(+)-Asparagine Monohydrate
Catalog No.:BCC8332
CAS No.:5794-13-8
- Officinalisinin I
Catalog No.:BCN2825
CAS No.:57944-18-0
- Bax inhibitor peptide V5
Catalog No.:BCC2394
CAS No.:579492-81-2
- Bax inhibitor peptide P5
Catalog No.:BCC2393
CAS No.:579492-83-4
- Caffeine
Catalog No.:BCN5807
CAS No.:58-08-2
- Pyrimethamine
Catalog No.:BCC2307
CAS No.:58-14-0
- Aminophenazone
Catalog No.:BCC8815
CAS No.:58-15-1
Draft Genome Sequence of Streptomyces fradiae olg1-1, a Strain Resistant to Nitrone-Oligomycin.[Pubmed:26494685]
Genome Announc. 2015 Oct 22;3(5). pii: 3/5/e01252-15.
We report a draft genome sequence of Streptomyces fradiae olg1-1, a mutant strain derived from the model object S. fradiae ATCC 19609, which is resistant to nitrone-Oligomycin And has a mutation in the DNA-binding domain of a transcriptional regulator PadR.
Underestimation of the Maximal Capacity of the Mitochondrial Electron Transport System in Oligomycin-Treated Cells.[Pubmed:26950698]
PLoS One. 2016 Mar 7;11(3):e0150967.
The maximal capacity of the mitochondrial electron transport system (ETS) in intact cells is frequently estimated by promoting protonophore-induced maximal oxygen consumption preceded by inhibition of oxidative phosphorylation by oligomycin. In the present study, human glioma (T98G and U-87MG) and prostate cancer (PC-3) cells were titrated with different concentrations of the protonophore CCCP to induce maximal oxygen consumption rate (OCR) within respirometers in a conventional growth medium. The results demonstrate that the presence of oligomycin or its A-isomer leads to underestimation of maximal ETS capacity. In the presence of oligomycin, the spare respiratory capacity (SRC), i.e., the difference between the maximal and basal cellular OCR, was underestimated by 25 to 45%. The inhibitory effect of oligomycin on SRC was more pronounced in T98G cells and was observed in both suspended and attached cells. Underestimation of SRC also occurred when oxidative phosphorylation was fully inhibited by the ATP synthase inhibitor citreoviridin. Further experiments indicated that oligomycin cannot be replaced by the adenine nucleotide translocase inhibitors bongkrekic acid or carboxyatractyloside because, although these compounds have effects in permeabilized cells, they do not inhibit oxidative phosphorylation in intact cells. We replaced CCCP by FCCP, another potent protonophore and similar results were observed. Lower maximal OCR and SRC values were obtained with the weaker protonophore 2,4-dinitrophenol, and these parameters were not affected by the presence of oligomycin. In permeabilized cells or isolated brain mitochondria incubated with respiratory substrates, only a minor inhibitory effect of oligomycin on CCCP-induced maximal OCR was observed. We conclude that unless a previously validated protocol is employed, maximal ETS capacity in intact cells should be estimated without oligomycin. The inhibitory effect of an ATP synthase blocker on potent protonophore-induced maximal OCR may be associated with impaired metabolism of mitochondrial respiratory substrates.
Functional characterization of CYP107W1 from Streptomyces avermitilis and biosynthesis of macrolide oligomycin A.[Pubmed:25849761]
Arch Biochem Biophys. 2015 Jun 1;575:1-7.
Streptomyces avermitilis contains 33 cytochrome P450 genes in its genome, many of which play important roles in the biosynthesis process of antimicrobial agents. Here, we characterized the biochemical function and structure of CYP107W1 from S. avermitilis, which is responsible for the 12-hydroxylation reaction of oligomycin C. CYP107W1 was expressed and purified from Escherichia coli. Purified proteins exhibited the typical CO-binding spectrum of P450. Interaction of oligomycin C and Oligomycin A (12-hydroxylated oligomycin C) with purified CYP107W1 resulted in a type I binding with Kd values of 14.4 +/- 0.7 muM and 2.0 +/- 0.1 muM, respectively. LC-mass spectrometry analysis showed that CYP107W1 produced Oligomycin A by regioselectively hydroxylating C12 of oligomycin C. Steady-state kinetic analysis yielded a kcat value of 0.2 min(-1) and a Km value of 18 muM. The crystal structure of CYP107W1 was determined at 2.1 A resolution. The overall P450 folding conformations are well conserved, and the open access binding pocket for the large macrolide oligomycin C was observed above the distal side of heme. This study of CYP107W1 can help a better understanding of clinically important P450 enzymes as well as their optimization and engineering for synthesizing novel antibacterial agents and other pharmaceutically important compounds.
Structural Analysis of the Streptomyces avermitilis CYP107W1-Oligomycin A Complex and Role of the Tryptophan 178 Residue.[Pubmed:26883908]
Mol Cells. 2016 Mar;39(3):211-6.
CYP107W1 from Streptomyces avermitilis is a cytochrome P450 enzyme involved in the biosynthesis of macrolide Oligomycin A. A previous study reported that CYP107W1 regioselectively hydroxylated C12 of oligomycin C to produce Oligomycin A, and the crystal structure of ligand free CYP107W1 was determined. Here, we analyzed the structural properties of the CYP107W1-Oligomycin A complex and characterized the functional role of the Trp178 residue in CYP107W1. The crystal structure of the CYP107W1 complex with Oligomycin A was determined at a resolution of 2.6 A. Oligomycin A is bound in the substrate access channel on the upper side of the prosthetic heme mainly by hydrophobic interactions. In particular, the Trp178 residue in the active site intercalates into the large macrolide ring, thereby guiding the substrate into the correct binding orientation for a productive P450 reaction. A Trp178 to Gly mutation resulted in the distortion of binding titration spectra with Oligomycin A, whereas binding spectra with azoles were not affected. The Gly178 mutant's catalytic turnover number for the 12-hydroxylation reaction of oligomycin C was highly reduced. These results indicate that Trp178, located in the open pocket of the active site, may be a critical residue for the productive binding conformation of large macrolide substrates.
Apoptolidin, a selective cytotoxic agent, is an inhibitor of F0F1-ATPase.[Pubmed:11182320]
Chem Biol. 2001 Jan;8(1):71-80.
BACKGROUND: Apoptolidin is a macrolide originally identified on the basis of its ability to selectively kill E1A and E1A/E1B19K transformed rat glial cells while not killing untransformed glial cells. The goal of this study was to identify the molecular target of this newly discovered natural product. RESULTS: Our approach to uncovering the mechanism of action of apoptolidin utilized a combination of molecular and cell-based pharmacological assays as well as structural comparisons between apoptolidin and other macrocyclic polyketides with known mechanism of action. Cell killing induced by apoptolidin was independent of p53 status, inhibited by BCL-2, and dependent on the action of caspase-9. PARP was completely cleaved in the presence of 1 microM apoptolidin within 6 h in a mouse lymphoma cell line. Together these results suggested that apoptolidin might target a mitochondrial protein. Structural comparisons between apoptolidin and other macrolides revealed significant similarity between the apoptolidin aglycone and oligomycin, a known inhibitor of mitochondrial F0F1-ATP synthase. The relevance of this similarity was established by demonstrating that apoptolidin is a potent inhibitor of the F0F1-ATPase activity in intact yeast mitochondria as well as Triton X-100-solubilized ATPase preparations. The K(i) for apoptolidin was 4-5 microM. The selectivity of apoptolidin in the NCI-60 cell line panel was found to correlate well with that of several known anti-fungal natural products that inhibit the eukaryotic mitochondrial F0F1-ATP synthase. SIGNIFICANCE: Although the anti-fungal activities of macrolide inhibitors of the mitochondrial F0F1-ATP synthase such as oligomycin, ossamycin and cytovaricin are well-documented, their unusual selectivity toward certain cell types is not widely appreciated. The recent discovery of apoptolidin, followed by the demonstration that it is an inhibitor of the mitochondrial F0F1-ATP synthase, highlights the potential relevance of these natural products as small molecules to modulate apoptotic pathways. The mechanistic basis for selective cytotoxicity of mitochondrial ATP synthase inhibitors is discussed.


