Notoginsenoside SCAS# 575446-95-6 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 575446-95-6 | SDF | Download SDF |
| PubChem ID | 21674165 | Appearance | White crystalline powder |
| Formula | C63H106O30 | M.Wt | 1343.5 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
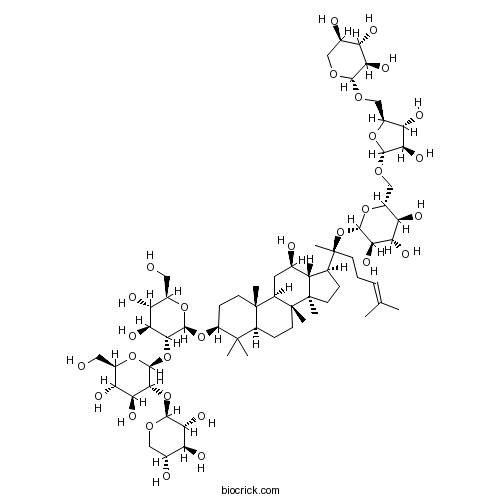
| Chemical Name | (2S,3R,4S,5S,6R)-2-[(2S)-2-[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-3-[(2R,3R,4S,5S,6R)-3-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxyoxan-2-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-12-hydroxy-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-methylhept-5-en-2-yl]oxy-6-[[(2R,3R,4R,5S)-3,4-dihydroxy-5-[[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxymethyl]oxolan-2-yl]oxymethyl]oxane-3,4,5-triol | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CCC4C3(CCC(C4(C)C)OC5C(C(C(C(O5)CO)O)O)OC6C(C(C(C(O6)CO)O)O)OC7C(C(C(CO7)O)O)O)C)C)O)C)OC8C(C(C(C(O8)COC9C(C(C(O9)COC1C(C(C(CO1)O)O)O)O)O)O)O)O)C | ||
| Standard InChIKey | AZIGQTILUNTIQH-RJLRDNOXSA-N | ||
| Standard InChI | InChI=1S/C63H106O30/c1-25(2)10-9-14-63(8,93-56-50(81)44(75)42(73)32(89-56)24-85-54-49(80)43(74)33(88-54)23-84-53-47(78)38(69)28(67)21-82-53)26-11-16-62(7)37(26)27(66)18-35-60(5)15-13-36(59(3,4)34(60)12-17-61(35,62)6)90-57-51(45(76)40(71)30(19-64)86-57)92-58-52(46(77)41(72)31(20-65)87-58)91-55-48(79)39(70)29(68)22-83-55/h10,26-58,64-81H,9,11-24H2,1-8H3/t26-,27+,28+,29+,30+,31+,32+,33-,34-,35+,36-,37-,38-,39-,40+,41+,42+,43-,44-,45-,46-,47+,48+,49+,50+,51+,52+,53-,54+,55-,56-,57-,58-,60-,61+,62+,63-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Notoginsenoside S may have hepatoprotective effects. |
| Structure Identification | Journal of Natural Products, 2003, 66(7):922-927.Structures of new dammarane-type Triterpene Saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal Ginseng Saponins.[Reference: WebLink]The saponin fraction from the flower buds of Panax notoginseng exhibited protective effect on liver injury induced by d-galactosamine and lipopolysaccharide.
|

Notoginsenoside S Dilution Calculator

Notoginsenoside S Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.7443 mL | 3.7216 mL | 7.4432 mL | 14.8865 mL | 18.6081 mL |
| 5 mM | 0.1489 mL | 0.7443 mL | 1.4886 mL | 2.9773 mL | 3.7216 mL |
| 10 mM | 0.0744 mL | 0.3722 mL | 0.7443 mL | 1.4886 mL | 1.8608 mL |
| 50 mM | 0.0149 mL | 0.0744 mL | 0.1489 mL | 0.2977 mL | 0.3722 mL |
| 100 mM | 0.0074 mL | 0.0372 mL | 0.0744 mL | 0.1489 mL | 0.1861 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-His(Nτ-Me)-OMe.2HCl
Catalog No.:BCC2958
CAS No.:57519-09-2
- 2-Methylsulfanylpyrimidin-4(3H)-one
Catalog No.:BCC8582
CAS No.:5751-20-2
- 6-Benzyloxypurine
Catalog No.:BCC8770
CAS No.:57500-07-9
- 3-Benzalphthalide
Catalog No.:BCC8621
CAS No.:575-61-1
- 3,4'-Di-O-methylellagic acid
Catalog No.:BCN3710
CAS No.:57499-59-9
- Carpachromene
Catalog No.:BCN5779
CAS No.:57498-96-1
- Angeflorin
Catalog No.:BCN6656
CAS No.:57498-69-8
- Carbasalate calcium
Catalog No.:BCC8904
CAS No.:5749-67-7
- BRL-54443
Catalog No.:BCC5047
CAS No.:57477-39-1
- 16-Deoxysaikogenin F
Catalog No.:BCN6414
CAS No.:57475-62-4
- Ibuprofen Lysine
Catalog No.:BCC2547
CAS No.:57469-77-9
- H-Asn-OMe.HCl
Catalog No.:BCC2876
CAS No.:57461-34-4
- SNOG
Catalog No.:BCC6714
CAS No.:57564-91-7
- Isofuranodiene
Catalog No.:BCN5781
CAS No.:57566-47-9
- PNU 37883 hydrochloride
Catalog No.:BCC7262
CAS No.:57568-80-6
- (3beta,24xi)-Cycloartane-3,24,25-triol
Catalog No.:BCN5782
CAS No.:57576-29-1
- (24S)-Cycloartane-3,24,25-triol 24,25-acetonide
Catalog No.:BCN1414
CAS No.:57576-31-5
- (2,4-Dihydroxyphenyl)acetonitrile
Catalog No.:BCN5783
CAS No.:57576-34-8
- Norcepharadione B
Catalog No.:BCN5784
CAS No.:57576-41-7
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
- Cycloartane-3,24,25-triol
Catalog No.:BCC8922
CAS No.:57586-98-8
- Biocytin
Catalog No.:BCC7659
CAS No.:576-19-2
- Piperenone
Catalog No.:BCN6578
CAS No.:57625-31-7
- Fenobam
Catalog No.:BCC7345
CAS No.:57653-26-6
Panax notoginsenoside saponins Rb1 regulates the expressions of Akt/ mTOR/PTEN signals in the hippocampus after focal cerebral ischemia in rats.[Pubmed:29501622]
Behav Brain Res. 2018 Jun 1;345:83-92.
Panax Notoginsenoside Saponins Rb1 (PNS-Rb1) is an important active ingredient of panax notoginseng for effective treatment of cerebrovascular diseases. However, the mechanism underlying its actions in the state of cerebral ischemia is still unclear. We asked whether the potential neuroprotection of PNS-Rb1 on the brain is due to, at least partially, its modulation of AkT/mTOR/PTEN signalling pathway along with down-regulation of caspase-3 in rats subjected to phototrombic stroke. To test this hypothesis, rats with induced photothrombotic stroke were treated with PNS-Rb1 (applied in three different doses, 25mg/kg, 50mg/kg,100mg/kg, respectively) or saline, while sham operated rats injected with saline were used as the control. Our results indicate that PNS-Rb1 significantly alleviated the morphological lesion concomitant with improvement of cognitive and sensorimotor deficits induced by ischemic stroke. Moreover, immunohistochemistry and Western blot analyses showed that PNS Rb1 in a dose dependent manner increased the expressions of P-Akt, P-mTOR and reduced P-PTEN and caspase-3. The present study suggests that the improvement of cognitive and sensorimotor deficits by PNS-Rb1 is made, at least partially, by the modulation of the Akt/mTOR/PTEN signalling pathway.
Combination of ginsenoside Rb1 and Rd protects the retina against bright light-induced degeneration.[Pubmed:28729651]
Sci Rep. 2017 Jul 20;7(1):6015.
Photoreceptor degeneration is a central pathology of various retinal degenerative diseases which currently lack effective therapies. Antioxidant and anti-inflammatory activities are noted for Panax Notoginsenoside Saponins (PNS) and related saponin compound(s). However, the photoreceptor protective potentials of PNS or related saponin compound(s) remain unknown. The current study revealed that PNS protected against photoreceptor loss in bright light-exposed BALB/c mice. Combination of ginsenoside Rb1 and Rd, two major saponin compounds of PNS, recapitulated the retinal protection of PNS and attenuated retinal oxidative stress and inflammatory changes. Rb1 or Rd partially alleviated all-trans-Retinal-induced oxidative stress in ARPE19 cells. Rb1 or Rd suppressed lipopolysaccharides (LPS)-induced proinflammatory gene expression in ARPE19 and RAW264.7 cells. Rb1 or Rd also modulated the expression of proinflammatory microRNA, miR-155 and its direct target, anti-inflammatory SHIP1, in LPS-stimulated RAW264.7 cells. The retinal expression of miR-155 and SHIP1 was altered preceding extensive retinal damage, which was maintained at normal level by Rb1 and Rd combination. This work shows for the first time that altered expression of miR-155 and SHIP1 are involved in photoreceptor degeneration. Most importantly, novel retinal protective activities of combination of Rb1 and Rd justify further evaluation for the treatment of related retinal degenerative disorders.


