NPE-caged-HPTSCAS# 223759-19-1 |
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Cilnidipine
Catalog No.:BCC1083
CAS No.:132203-70-4
- Pregabalin
Catalog No.:BCN2175
CAS No.:148553-50-8
- NNC 55-0396
Catalog No.:BCC1803
CAS No.:357400-13-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 223759-19-1 | SDF | Download SDF |
| PubChem ID | 56972217 | Appearance | Powder |
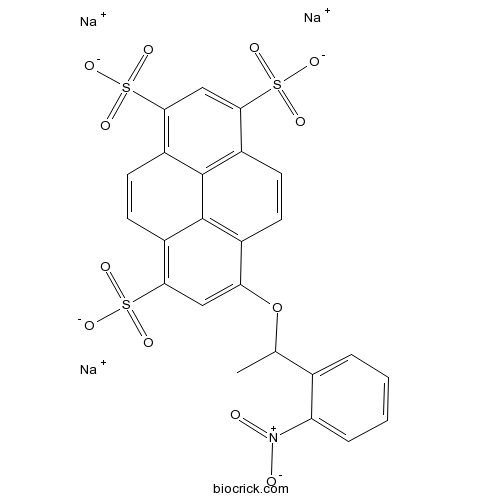
| Formula | C24H14NO12Na3S3 | M.Wt | 673.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
| Chemical Name | trisodium;8-[1-(2-nitrophenyl)ethoxy]pyrene-1,3,6-trisulfonate | ||
| SMILES | CC(C1=CC=CC=C1[N+](=O)[O-])OC2=CC(=C3C=CC4=C(C=C(C5=C4C3=C2C=C5)S(=O)(=O)[O-])S(=O)(=O)[O-])S(=O)(=O)[O-].[Na+].[Na+].[Na+] | ||
| Standard InChIKey | CRGABPHUJQWBOR-UHFFFAOYSA-K | ||
| Standard InChI | InChI=1S/C24H17NO12S3.3Na/c1-12(13-4-2-3-5-18(13)25(26)27)37-19-10-20(38(28,29)30)15-8-9-17-22(40(34,35)36)11-21(39(31,32)33)16-7-6-14(19)23(15)24(16)17;;;/h2-12H,1H3,(H,28,29,30)(H,31,32,33)(H,34,35,36);;;/q;3*+1/p-3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Caged fluorescent pH indicator. Rapidly releases the fluorophore HPTS (pyranine) (pKa 7.25) upon two-photon excitation (>3000 s-1). Routinely used for photolysis calibration. Suitable for use in small-compartment diffusion studies. |

NPE-caged-HPTS Dilution Calculator

NPE-caged-HPTS Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4847 mL | 7.4236 mL | 14.8471 mL | 29.6943 mL | 37.1179 mL |
| 5 mM | 0.2969 mL | 1.4847 mL | 2.9694 mL | 5.9389 mL | 7.4236 mL |
| 10 mM | 0.1485 mL | 0.7424 mL | 1.4847 mL | 2.9694 mL | 3.7118 mL |
| 50 mM | 0.0297 mL | 0.1485 mL | 0.2969 mL | 0.5939 mL | 0.7424 mL |
| 100 mM | 0.0148 mL | 0.0742 mL | 0.1485 mL | 0.2969 mL | 0.3712 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sodium Monensin
Catalog No.:BCC5319
CAS No.:22373-78-0
- 4-Methoxysalicylic acid
Catalog No.:BCN7783
CAS No.:2237-36-7
- Eupatilin
Catalog No.:BCN2336
CAS No.:22368-21-4
- Mirabegron (YM178)
Catalog No.:BCC3814
CAS No.:223673-61-8
- Collagen proline hydroxylase inhibitor
Catalog No.:BCC1494
CAS No.:223666-07-7
- Collagen proline hydroxylase inhibitor-1
Catalog No.:BCC1495
CAS No.:223663-32-9
- K-115 free base
Catalog No.:BCC5501
CAS No.:223645-67-8
- Tiadinil
Catalog No.:BCC8070
CAS No.:223580-51-6
- CPA inhibitor
Catalog No.:BCC1500
CAS No.:223532-02-3
- YM 58483
Catalog No.:BCC7817
CAS No.:223499-30-7
- ONO 4817
Catalog No.:BCC2375
CAS No.:223472-31-9
- GLP-2 (human)
Catalog No.:BCC5891
CAS No.:223460-79-5
- Bestatin trifluoroacetate
Catalog No.:BCC3909
CAS No.:223763-80-2
- Pedalitin
Catalog No.:BCN3954
CAS No.:22384-63-0
- Serratenediol
Catalog No.:BCN5061
CAS No.:2239-24-9
- 3,8-Di-O-methylellagic acid
Catalog No.:BCN5062
CAS No.:2239-88-5
- Aristola-1(10),8-dien-2-one
Catalog No.:BCN7608
CAS No.:22391-34-0
- Cyclobuxine D
Catalog No.:BCC9221
CAS No.:2241-90-9
- 3-Epiturraeanthin
Catalog No.:BCN5063
CAS No.:22415-24-3
- Siramesine hydrochloride
Catalog No.:BCC5134
CAS No.:224177-60-0
- Incensole
Catalog No.:BCN3831
CAS No.:22419-74-5
- 2,2'-Anhydro-5-methyluridine
Catalog No.:BCC8486
CAS No.:22423-26-3
- Bayogenin methyl ester
Catalog No.:BCN3722
CAS No.:22425-81-6
- Ginsenoside Rg1
Catalog No.:BCN1066
CAS No.:22427-39-0
Laser photolysis of caged compounds at 405 nm: photochemical advantages, localisation, phototoxicity and methods for calibration.[Pubmed:19427524]
J Neurosci Methods. 2009 May 30;180(1):9-21.
Rapid, localised photolytic release of neurotransmitters from caged precursors at synaptic regions in the extracellular space is greatly hampered at irradiation wavelengths in the near-UV, close to the wavelength of maximum absorption of the caged precursor, because of inner-filtering by strong absorption of light in the cage solution between the objective and cell. For this reason two-photon excitation is commonly used for photolysis, particularly at multiple points distributed over large fields; or, with near-UV, if combined with local perfusion of the cage. These methods each have problems: the small cross-sections of common cages with two-photon excitation require high cage concentrations and light intensities near the phototoxic limit, while local perfusion gives non-uniform cage concentrations over the field of view. Single-photon photolysis at 405 nm, although less efficient than at 330-350 nm, with present cages is more efficient than two-photon photolysis. The reduced light absorption in the bulk cage solution permits efficient wide-field uncaging at non-toxic intensities with uniform cage concentration. Full photolysis of MNI-glutamate with 100 micros pulses required intensities of 2 mW microm(-2) at the preparation, shown to be non-toxic with repeated exposures. Light scattering at 405 nm was estimated as 50% at 18 microm depth in 21-day rat cerebellum. Methods are described for: (1) varying the laser spot size; (2) photolysis calibration in the microscope with the caged fluorophore NPE-HPTS over the wavelength range 347-405 nm; and (3) determining the point-spread function of excitation. Furthermore, DM-Nitrophen photolysis at 405 nm was efficient for intracellular investigations of Ca2+-dependent processes.
Neuronal activity regulates diffusion across the neck of dendritic spines.[Pubmed:16272125]
Science. 2005 Nov 4;310(5749):866-9.
In mammalian excitatory neurons, dendritic spines are separated from dendrites by thin necks. Diffusion across the neck limits the chemical and electrical isolation of each spine. We found that spine/dendrite diffusional coupling is heterogeneous and uncovered a class of diffusionally isolated spines. The barrier to diffusion posed by the neck and the number of diffusionally isolated spines is bidirectionally regulated by neuronal activity. Furthermore, coincident synaptic activation and postsynaptic action potentials rapidly restrict diffusion across the neck. The regulation of diffusional coupling provides a possible mechanism for determining the amplitude of postsynaptic potentials and the accumulation of plasticity-inducing molecules within the spine head.
The efficiency of two-photon photolysis of a "caged" fluorophore, o-1-(2-nitrophenyl)ethylpyranine, in relation to photodamage of synaptic terminals.[Pubmed:11908850]
Eur Biophys J. 2002 Feb;30(8):588-604.
Localized photolysis of caged neurotransmitters with the two-photon effect for investigations at synaptic preparations was evaluated by determining the toxicity to synaptic transmission of pulsed near-IR laser light focused into the terminals of the snake neuromuscular junction, and measuring the extent of photolysis of a conventional caging group with similar irradiation in microcuvette experiments. Photodamage was seen in synaptic terminals as a large, irreversible increase of spontaneous synaptic activity with laser flashes of 5 ms at 1 Hz at average powers > 5 mW and was due to multiphoton absorption. Localized photolysis due to two-photon absorption was investigated for a representative caged fluorophore, the 1-(2-nitrophenyl)ethyl ether of pyranine (NPE-HPTS). Irradiation of NPE-HPTS at 5 mW with the same optical arrangement produced very low rates of photolysis. NPE-HPTS photolysis mechanisms were investigated at high laser powers by measuring (1) the kinetics of two-photon fluorescence generated by two-photon photolysis in the focal volume and (2) the rates of HPTS accumulation inside closed 2-10 microm radius vesicles, measured with one-photon excitation during two-photon photolysis by repetitive 10 micros laser exposures. The two-photon crosssection of NPE-HPTS photolysis calculated from the rates is 0.02-0.04 GM (10(-50) cm4 x s/photon) and limits the efficiency of photolysis at 5 mW. With free diffusional exchange, 50% steady-state cage depletion in the focal volume was estimated to occur only at high laser powers of ca. 72 mW, masked in experiments by multiphoton bleaching. Based on these results, the two-photon photolysis cross-section needed for 50% steady-state photolysis of a caged neurotransmitter at 5 mW is calculated as 31 GM, much higher than in existing caged compounds.
Chemotactic responses of Escherichia coli to small jumps of photoreleased L-aspartate.[Pubmed:10049350]
Biophys J. 1999 Mar;76(3):1706-19.
Computer-assisted motion analysis coupled to flash photolysis of caged chemoeffectors provides a means for time-resolved analysis of bacterial chemotaxis. Escherichia coli taxis toward the amino acid attractant L-aspartate is mediated by the Tar receptor. The physiology of this response, as well as Tar structure and biochemistry, has been studied extensively. The beta-2, 6-dinitrobenzyl ester of L-aspartic acid and the 1-(2-nitrophenyl)ethyl ether of 8-hydroxypyrene-1,3,6-tris-sulfonic acid were synthesized. These compounds liberated L-aspartate and the fluorophore 8-hydroxypyrene 1,3,6-tris-sulfonic acid (pyranine) upon irradiation with near-UV light. Photorelease of the fluorophore was used to define the amplitude and temporal stability of the aspartate jumps employed in chemotaxis experiments. The dependence of chemotactic adaptation times on aspartate concentration, determined in mixing experiments, was best fit by two Tar aspartate-binding sites. Signal processing (excitation) times, amplitudes, and adaptive recovery of responses elicited by aspartate jumps producing less than 20% change in receptor occupancy were characterized in photorelease assays. Aspartate concentration jumps in the nanomolar range elicited measurable responses. The response threshold and sensitivity of swimming bacteria matched those of bacteria tethered to glass by a single flagellum. Stimuli of similar magnitude, delivered either by rapid mixing or photorelease, evoked responses of similar strength, as assessed by recovery time measurements. These times remained proportional to change in receptor occupancy close to threshold, irrespective of prior occupancy. Motor excitation responses decayed exponentially with time. Rates of excitation responses near threshold ranged from 2 to 7 s-1. These values are consistent with control of excitation signaling by decay of phosphorylated pools of the response regulator protein, CheY. Excitation response rates increased slightly with stimulus size up to values limited by the instrumentation; the most rapid was measured to be 16 +/- 3 (SE) s-1. This increase may reflect simultaneous activation of CheY dephosphorylation, together with inhibition of its phosphorylation.
Comparison of simultaneous pH measurements made with 8-hydroxypyrene-1,3,6-trisulphonic acid (HPTS) and pH-sensitive microelectrodes in snail neurones.[Pubmed:9683736]
Pflugers Arch. 1998 Jul;436(4):615-22.
We have evaluated the pyrene-based ratiometric fluorescent dye, 8-hydroxypyrene-1,3,6-trisulphonic acid (HPTS), by using it in conjunction with glass pH-sensitive microelectrodes to measure intracellular pH (pHi) in voltage-clamped snail neurones. Intracellular acidification with propionic acid, and alkalinization following the activation of H+ channels allowed the calibration of the dye to be compared with that of the pH microelectrode over the pH range 6.50-7.50. HPTS calibrated in vitro and glass pH-sensitive microelectrodes produced similar absolute resting pHi values, 7. 16+/-0.05 (n=10) and 7.17+/-0.06 (n=9) respectively in nominally CO2/HCO3--free saline. At both extremes of the pH range there were small discrepancies. At acidic pHi, 6.87+/-0.09 (n=5), the intracellular HPTS measurement differed by -0.08+/-0.03 pH units from the pH-sensitive microelectrode measurement. At alkaline pHi, 7. 32+/-0.10 (n=5), HPTS measurements produced pH values that differed by +0.07+/-0.04 pH units from those of the pH-sensitive microelectrode. Some of the discrepancy could be accounted for by the slow response of the recessed-tip pH-sensitive microelectrode (time constant 77+/-15 s, n=3). Further experiments showed that HPTS, used at an intracellular concentration of 200 microM to 2 mM, did not block activity-dependent pHi changes. The intracellular HPTS concentration was calculated by measurement of intracellular chloride during a series of HPTS-KCl injections. Comparison of HPTS with 2',7'-bis(2-carboxyethyl)-5,6-carboxyfluorescein (BCECF), at the same concentration, showed that HPTS produces a larger change in ratio over the pH range 6.00-8.00.


